Untreated condensate can plunge to pH ≲3 and pit steel with just a few ppb (parts per billion) of oxygen. A smart blend of neutralizing and filming amines plus a robust deaerator flips the script.
Industry: Textile | Process: Boiler_&_Steam_Distribution
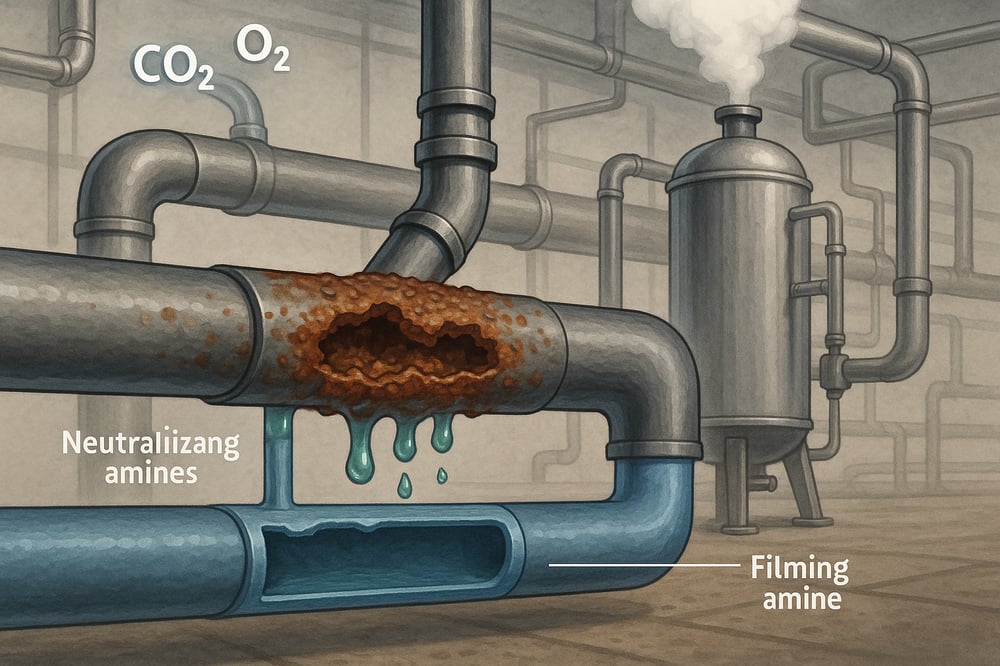
In a textile mill’s steam maze, acidic condensate is a quiet balance-sheet leak. Dissolved CO₂ from makeup water becomes carbonic acid (H₂CO₃), driving condensate pH as low as ~3, where iron dissolves via Fe + 2H⁺ → Fe²⁺ + H₂ (hydrogen gas) (pubs.rsc.org; www.watertechnologies.com). Even trace dissolved oxygen (DO) drives severe pitting on carbon steel (pubs.rsc.org), and experts warn condensate O₂ pitting is “particularly severe” (www.watertechnologies.com).
With kilometers of steam piping, failures mean downtime, hazardous leaks of “liquid gold,” and lost heat. Textile mills can break the cycle with three anchors: neutralizing amines to lift pH, filming amines to shield metal, and a hard-working deaerator to strip O₂ and CO₂.
Baca juga: Nutrient
Condensate chemistry and corrosion
Carbonic acid forms when CO₂ dissolves in water; at low pH, hydrogen ions attack iron rapidly. Below about pH 3, corrosion accelerates (pubs.rsc.org). Dissolved oxygen, even at a few ppb, localizes attack into pits (pubs.rsc.org; www.watertechnologies.com). The outcome of leaving condensate acidic and oxygenated is predictable: rapid steel thinning and leaks.
Neutralizing amines for pH control
Neutralizing amines are volatile bases dosed to the boiler/steam/condensate cycle to raise condensate pH (a measure of acidity/alkalinity). They hydrolyze to form hydroxide: “R–NH₂ + H₂O → R–NH₃⁺ + OH⁻,” which neutralizes carbonic acid via “H₂CO₃ + OH⁻ → HCO₃⁻ + H₂O” (www.watertechnologies.com; www.watertechnologies.com). In mixed-metal systems (iron and copper), guidance targets condensate pH ≈8.8–9.2 (www.watertechnologies.com), while Indonesian boiler standards specify feedwater pH 8.5–9.6 at 25°C (id.scribd.com).
In practice, mills lean on blends of neutralizing amines such as morpholine, cyclohexylamine (CHA), and diethylaminoethanol (DEAE) (www.chemaqua.com; www.watertechnologies.com). Effectiveness depends on volatility: at atmospheric pressure, morpholine’s vapor/liquid distribution is ~0.4, while CHA’s is ~4.0 (fr.scribd.com). A typical strategy blends a lower-volatility amine (morpholine) with a more volatile amine (CHA or DEAE) so protection reaches all condensation points as temperatures fall (fr.scribd.com).
Neutralizing capacity tracks molecular weight: DEAE neutralizes ~2.6× its weight in CO₂, CHA ~2.3×, morpholine ~2.0× (Veolia table; www.watertechnologies.com). Operators simply feed to hold condensate near pH ~9.0; underfeeding or bypassing rapidly returns the system to low pH and high corrosion.
Done right, pH control preserves protective magnetite films and slashes metal release. Power-plant benchmarks for all-volatile treatment (AVT(R), a reducing program using only volatile chemicals) cite feedwater iron [Fe] < 5 µg/L and copper [Cu] < 2 µg/L, with AVT(O) (oxidizing) delivering Fe < 2 µg/L (fliphtml5.com; fliphtml5.com). Textile systems run lower pressure, but the principle holds: raising condensate to ≈9 virtually eliminates carbonic-acid corrosion compared to untreated pH <3 that “fail[s]” pipelines (pubs.rsc.org).
Baca juga: Neutralizing Amine
Filming amines as hydrophobic barriers
Filming (film-forming) amines act like a one-molecule-thick raincoat. The nitrogen “head” bonds to steel while the long hydrophobic “tail” faces outward, repelling water and dissolved gases (www.chemaqua.com). On treated pipe, condensate beads like droplets on wax; operators confirm film presence with barrel or spool-piece checks (www.chemaqua.com).
These corrosion inhibitors protect “blind spots” where flow is sluggish and bulk pH may dip. Many long-chain amines (e.g., dodecylamine, octadecylamine) and proprietary blends are used; several are slightly basic and consume some carbonic acid during condensation. Field studies in power plants report significant reductions in localized attack even with marginal pH control, and EPRI notes “significant protection” in low-pressure phase transitions (www.ccj-online.com; www.ccj-online.com).
Use typically starts with slow feed into the condensate return header, often alongside neutralizers, to avoid dislodging rust/scale; beading checks confirm coverage (www.chemaqua.com). Many programs deliberately pair neutralizing plus filming amines, sometimes managed by an F/N ratio tuned to the system.
Baca juga: Nutrient Removal
Mechanical deaeration for O₂ control
A robust deaerator is essential. By heating and venting per Henry’s Law (gas solubility proportional to partial pressure), spray- or tray-type units strip dissolved gases from feedwater (www.watertechnologies.com). As temperature rises, O₂ solubility plummets: at 25°C and 1 atm, DO is ≈8 mg/L; at 100°C (0.2 atm O₂) it’s ≈8 ppm, and at 90–100°C under vacuum it can drop to ~4 ppm (0.1 atm O₂) (www.watertechnologies.com). Modern units heat to ~95–100°C and vent effectively, achieving ~95–99% O₂ removal with downstream levels in the few-ppb range.
Targets are tight. Mechanical deaerators can approach ~0.005 mg/L (5 ppb) DO (policy.asiapacificenergy.org), and Indonesia’s SNI-7268 expects essentially zero (≲0.007 mg/L) in feedwater (id.scribd.com). Deaeration also vents CO₂ from thermal bicarbonate breakdown, cutting carbonic acid formation (www.watertechnologies.com).
Best practice pairs the deaerator with chemical scavengers to polish out residual ppb oxygen, because even 5–10 ppb can initiate pits. Programs use hydrazine, sodium sulfite, or organic scavengers, and—per an Indonesian handbook—“good practice requires removal of that trace oxygen with a chemical scavenger” (policy.asiapacificenergy.org; id.scribd.com). Many mills specify oxygen scavengers directly in their boiler chemical programs.
Outcome: with proper deaeration and scavenging, DO routinely measures <0.01 ppm; operators report ORP (oxidation–reduction potential) around -25 mV and single-ppb iron in well-controlled feedwater (fliphtml5.com; id.scribd.com; fliphtml5.com). A single vacuum leak, however, can undo removal efficacy.
Baca juga: Oil Removal
Operations and energy economics
Returning hot condensate conserves fuel—industry notes it still holds ~15% of the boiler’s input energy after heat exchange (www.filtox.com). Chem-Aqua’s rule of thumb: “every gallon of condensate returned… saves a cubic foot of natural gas,” about 4.2 MJ per gallon (www.chemaqua.com). One report calculates recycling each tonne of condensate avoids ~0.95 tonnes of CO₂ emissions (www.filtox.com).
Textile mills run heavy steam loads for dyeing and drying (www.filtox.com). Raising condensate to ~pH 9 and removing O₂ trims corrosion from ~0.1–1.0 mm/year into the μm/year range, avoiding failures linked to untreated condensate often <pH 3 (pubs.rsc.org). Preventing a single significant leak can save thousands of dollars annually; a Bangladesh case study reported 694 GJ/day losses systemwide, implying a 10% improvement from condensate recovery worth ~69.4 GJ/day (link.springer.com).
Baca juga: Oxygen/H2S Scavengers
Standards and compliance benchmarks
Indonesia’s SNI-7268 (2009) sets strict feedwater limits—pH, conductivity, and DO—mirroring Western practice. It effectively requires dissolved oxygen ≲0.007 mg/L and encourages “reducing substances (ammonia, hydrazine, volatile amines…)” for O₂ removal (id.scribd.com; id.scribd.com). National campaigns emphasize condensate reuse to save fuel. Meeting these specs—condensate pH ~9, DO ≈0—aligns with both technical guidance and regulatory expectations.
Baca juga: Poly Aluminum Chloride (PAC)
Implementation details and monitoring
Chemical regimen: Blend morpholine with CHA to hold condensate ~pH 9, feeding at the condensate return header or into deaerator suction; test amine levels via titration/field kits (www.watertechnologies.com; fr.scribd.com). Many programs formalize this under broader boiler chemical control. Dose an O₂ scavenger in the deaerator—hydrazine for high pressure; sulfite or organic for moderate (policy.asiapacificenergy.org).
Mechanicals: Keep the deaerator at ~95–100°C, maintain spray nozzles/trays and venting, and sample DO downstream—target <0.01 mg/L (policy.asiapacificenergy.org; id.scribd.com). Feed filming amines slowly to avoid dislodging deposits, and verify coverage with beading checks (www.chemaqua.com).
Monitoring: Track condensate pH (~8.8–9.2), DO (<0.01 mg/L), and corrosion coupons in returns; install spool samples where practical. Use an accurate dosing pump to maintain setpoints. Tepidity: ensure frequent blowdown if TDS from sulfite is high. Over months, document feedwater Fe/Cu trends and leak incidents; successful neutralizing-plus-filming-plus-deaeration programs often halve corrosion-product carryover and virtually eliminate pitting leaks.
Energy accounting: Chem-Aqua’s “every gallon returned saves a cubic foot of natural gas” underlines why condensate recovery multiplies benefits in mills with extensive steam usage (www.chemaqua.com; www.filtox.com). That’s in addition to the corrosion gains from pushing condensate toward pH ~9 and driving DO to near-zero with scavengers.
Baca juga: PAC Custom
Bottom line and sources
Strip oxygen aggressively with a robust deaerator and scavenger, neutralize CO₂ with volatile amines to raise condensate to pH ≈9, and supplement with a film-forming barrier; together, these steps can practically eliminate carbonic-acid corrosion, preserve equipment, and save substantial energy (policy.asiapacificenergy.org; id.scribd.com; www.watertechnologies.com; www.chemaqua.com; www.filtox.com; pubs.rsc.org).
Sources: Authoritative water-treatment and industry publications inform these figures—Veolia’s Water Handbook on condensate corrosion chemistry and treatment (www.watertechnologies.com; www.watertechnologies.com); textile condensate recovery benchmarks (www.filtox.com; www.filtox.com); and Indonesian standards (SNI) for feedwater O₂/pH limits (id.scribd.com; id.scribd.com).
