Ammonia/urea plants run recirculating cooling towers under extreme heat loads where minerals concentrate, ammonia and NOx can infiltrate, and biofilms thrive — a perfect storm for scale, corrosion, and Legionella. A coordinated program of scale inhibitors, steel and copper corrosion inhibitors, and dual biocides is how operators hold the line on heat transfer and reliability.
Industry: Fertilizer_(Ammonia_&_Urea) | Process: Process_Cooling_Systems
In a large ammonia/urea complex, untreated cooling water quickly “cycles up” as evaporation concentrates minerals and fouling takes hold, a dynamic that Power Engineering and Chemical Engineering have cataloged for decades. Even a thin carbonate layer can insulate condenser tubes, pushing fuel bills “hundreds to thousands of dollars per day” in a big plant (Power Engineering).
The risk profile is higher in fertilizer towers: ambient ammonia (NH₃) and NOx can enter the tower air and water (EPA archive; OSTI), where nitrifying bacteria oxidize NH₃ to nitrite/nitrate, lowering pH and forming corrosive acids (OSTI). In tropical settings (for example, Indonesia), warm 20–50 °C basins foster Legionella-bearing biofilms, as noted by beta.co.id.
The answer is a tightly coordinated chemical program — a blend of phosphonate and polymer scale inhibitors, azoles and film-formers for mixed metallurgy, plus a dual oxidizing/non-oxidizing biocide routine — of the type described in recent studies and industry guidance (MDPI; Chemical Processing).
Program overview and operating context
This design targets three failure modes at once: carbonate/sulfate scale, mixed‑metal corrosion, and microbiological growth. It aligns with cooling tower chemical programs used in heavy industry while addressing ammonia/urea‑specific contamination pathways.
Key terms used here: cycles of concentration (COC, the ratio of dissolved solids in recirculating water to make‑up), blowdown (controlled discharge to limit COC), Langelier and Ryznar indices (scaling/corrosion tendencies), ORP (oxidation‑reduction potential), HPC (heterotrophic plate count bacteria), Legionella (pneumonia‑causing bacteria), and MPY (mils per year, a corrosion rate unit).
Threshold and crystal‑distortion scale control
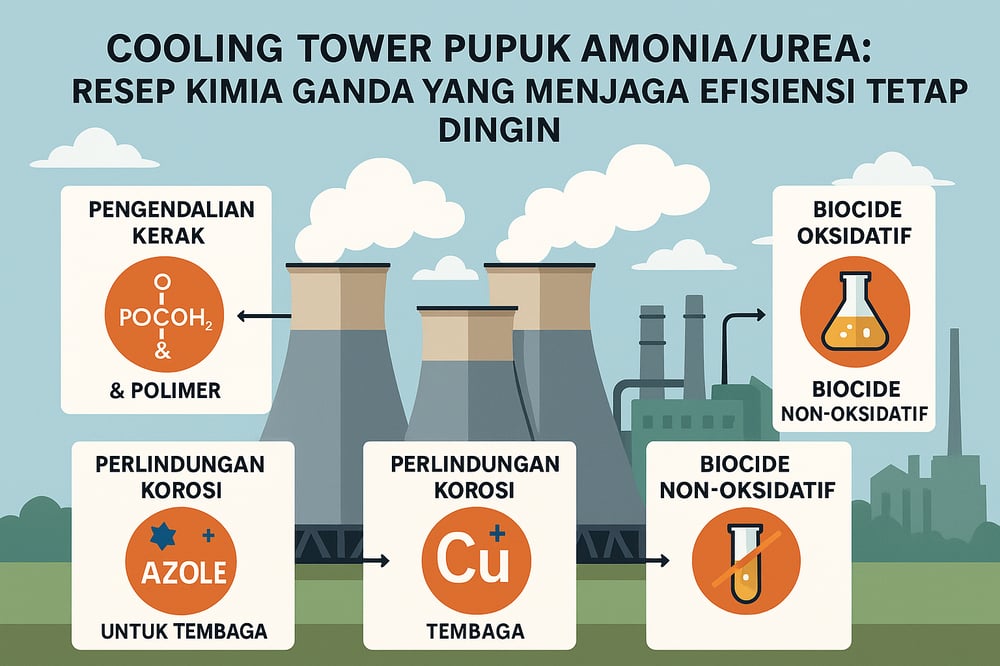
Scale control relies on threshold inhibition and crystal distortion. Operators continuously feed a synergistic blend of organophosphonates (e.g., HEDP, PBTC, phosphino‑succinates) with polymeric dispersants (e.g., polyacrylate, polyaspartate) so phosphonates chemisorb onto nascent CaCO₃ and polymers sequester Ca²⁺ and nuclei (MDPI).
Typical application rates are on the order of ~5–20 mg/L of each inhibitor; under severe fouling, higher levels (tens of mg/L) may be required. Static lab tests showed ~90 mg/L of a combined phosphonate/polymer blend achieved over 91% inhibition of CaCO₃ deposition (MDPI). A field‑trial formula with mass ratio phosphonate:PASP:PESA = 4:1:1 delivered 91.8% scale inhibition while reducing phosphorus residual by ~33% (MDPI), showing how “green” polymers (polymaleate, polyepoxysuccinate) can approach phosphonate performance with far less P‑loading.
In practice, tight pH control (roughly 7.5–9.0, depending on metallurgy) plus continuous metering keeps Langelier/Ryznar indices in a non‑scaling regime. That continuous metering is typically executed via accurate chemical feed equipment such as a dosing pump. Conductivity and hardness checks govern blowdown so impurities flush out before precipitation. With good control, common CaCO₃/CaSO₄/silicate scales remain suspended or deposit weakly, extending cleaning intervals and yielding a 30–50% reduction in fouling mass versus uncontrolled systems (Power Engineering; Chemical Engineering).
Operationally, the inhibitor blend maps directly to commercial offerings such as scale inhibitors for cooling towers combined with dispersant chemicals for particle control.
Mixed‑metallurgy corrosion protection
Cooling circuits span carbon steel piping, copper/brass or Cu–Ni tubes, and stainless components, so the program must protect each metal without creating galvanic hot spots.
For copper alloys, azole compounds (tolyltriazole or benzotriazole/BZT) at ~1–5 mg/L form a tight chemisorbed film on Cu/Cu₂O, stabilizing the protective Cu(I) oxide layer and discouraging conversion to less protective Cu(II) oxide (Veolia Water Technologies Handbook; Water Technology Report). These triazoles also bind dissolved Cu²⁺, limiting re‑plating onto steel (AZoM). Because oxidizers (chlorine, ozone) can degrade azole films, residuals are monitored (e.g., UV/Vis analysis) and replenished continuously (AZoM; Veolia Water Technologies Handbook).
For carbon steel, anodic film‑formers are used. Traditional nitrite or molybdate chemistries are options; in ammonia‑rich environments where bacteria can form nitrite/nitrate, organic alternatives (zinc‑free polyphosphates or polymers) are often preferred. Small amounts of zinc–molybdate (5–20 mg/L total) can deposit protective films (Water Technology Report), while modern programs frequently deploy polymeric filmers (polyaspartate, fatty amines) and maintain bulk pH ~8.5–9.0 to sustain alkaline passivation. Lab trials with advanced phosphonate/polymer blends at ~13 ppm total inhibitor delivered carbon‑steel corrosion ~2.1–2.3 MPY (WO2019005429A1).
Mixed‑metal precautions are explicit: even ppm‑level copper ions can plate onto steel and drive galvanic corrosion (AZoM), so blowdown and make‑up are watched for Cu (<0.2 ppm) and Fe(II) indicators; stainless sections avoid acid excursions (pH >7) and chloride issues. All inhibitors are fed continuously, with an initial “conditioning” dose on startup and maintenance doses at 20–50% of initial to preserve protective films (Water Technology Report). Commercially, this maps to blended corrosion inhibitors for mixed metallurgy in recirculating systems.
Dual biocide regimen and biofilm control

Warm, aerated tower water favors algae and bacteria, accelerating deposits and microbially influenced corrosion (MIC). The baseline is a dual program: continuous oxidizing sanitation plus regular non‑oxidizing shock, a combination recommended in industry practice (Midwest WT; Chemical Processing).
Continuous oxidizing biocide: maintain ~1–3 mg/L free chlorine (or 1–2 mg/L bromine) in the basin. Hypochlorite (bleach) or stabilized bromine are typical; alternatively, chlorine dioxide (ClO₂) at ~0.2–0.5 mg/L offers broad spectrum control. Oxidizers kill planktonic microbes and gradually penetrate biofilms, but require uninterrupted feed and monitoring (ORP ~600+ mV) to prevent regrowth (Chemical Processing).
Periodic non‑oxidizing shocks: once or twice weekly, dose glutaraldehyde at ~150–250 mg/L for several hours, or DBNPA (a brominated azole biocide) at ~50–100 mg/L. Alternating agents helps avoid resistance, consistent with AFL/ISO protocols (OSHA/CDC) cited by practitioners (Midwest WT; Chemical Processing). This approach routinely drives Legionella reductions >2 orders of magnitude in documented systems (MDPI; MDPI), with total plate counts (HPC) held low (e.g., <10³ CFU/mL).
Indonesia’s tropical climate intensifies vigilance: warm conditions favor Legionella, making reliable oxidizer residuals and periodic oxidative shocks critical (beta.co.id; Chemical Processing). Silicone‑based antifoam agents are added to suppress foam that can reduce cooling efficiency and aerosolize bacteria; organic additives are chosen for rapid biodegradation to meet effluent BOD/COD limits. These practices are directly supported by commercial biocide programs and antifoam formulations for towers.
Monitoring, setpoints, and outcomes
The combined program is tuned continuously with online meters and periodic lab tests. Operators trend conductivity (for COC), pH, corrosion coupon weight loss, inhibitor residuals, and HPC. As inhibitors prove effective (stable, deposit‑free surfaces), conductivity setpoints can rise to a COC of ~4–6 to save make‑up water. In one analogous case, moving from 3 to 6 cycles saved millions of liters annually without piping corrosion (Power Engineering; MDPI).
Program targets are explicit: stable heat‑transfer efficiency (cold‑water setpoint met), reduced corrosion rates (<2–3 MPY on steel; Cu ≪1 MPY), and biofouling under control (no detectable Legionella; HPC near background). All dosing is continuous — a fit for automated feed skids built around an accurate dosing pump — and aligns with local environmental safeguards by favoring low‑phosphorus, rapid‑degradation chemistries to meet Indonesian discharge limits (BOD/COD and metals), as discussed in MDPI and Chemical Engineering.
Why these tools, and why now
This chemistry stack reflects fundamentals and field data: the steep cost of untreated scale (Power Engineering), the shift toward polymer/phosphonate blends (Chemical Engineering; MDPI), dosed azoles for copper (1–3 ppm BZT; tolyltriazole similar or slightly higher if needed), zinc–molybdate for steel (5–20 mg/L total), and polymeric filmers that hold steel corrosion to low single‑digit MPY (2.1–2.3 MPY at ~13 ppm total inhibitor in lab trials) (Water Technology Report; Veolia Water Technologies Handbook; WO2019005429A1).
In short: a data‑driven, tightly dosed stack of cooling tower chemicals — scale inhibitors, dispersants, corrosion inhibitors, and biocides — is what lets ammonia/urea towers run hot, clean, and reliable under stress (Midwest WT; Chemical Processing).
