Modern wet scrubbers strip urea dust and ammonia from prilling and granulation air, routinely driving total particulate below 10 mg/Nm³ while recycling the captured nitrogen back into fertilizer streams. The payoff: cleaner stacks and higher yield, documented across recent Toyo, Stamicarbon, and thyssenkrupp projects.
Industry: Fertilizer_(Ammonia_&_Urea) | Process: Urea_Production
In urea prilling and granulation, the exhaust carries two revenue-leaking pollutants: fine urea dust and ammonia vapour. Operators are now designing scrubber systems that capture both, meet increasingly tight regulations, and feed the nitrogen straight back into product.
Typical regulatory targets sit in the tens of milligrams per normal cubic metre — mg/Nm³ (milligrams per normal cubic metre, a flow normalized to standard temperature and pressure). Industry guidelines often cite ≈50 mg/Nm³ for both urea (as particulate) and NH₃, according to BCInsight/CRU and Scribd. Indonesian law now requires continuous CEMS (continuous emissions monitoring systems) for particulate and NH₃ at urea plants (world.moleg.go.kr). In practice, operators aim well below those limits.
Recent projects report finished‑stack total particulate (TPM) below 10 mg/Nm³ with advanced scrubbing (BCInsight/CRU). At one large Indonesian plant (Kaltim No.5), a single‑stage water scrub met ~21 mg/Nm³ (no acid scrub was mandated) (BCInsight/CRU). By contrast, adding an acid scrub stage (e.g., at Nigeria’s 4,000 tpd plant) drove dust below 10 mg/Nm³ (BCInsight/CRU). Ammonia targets are similarly stringent (on the order of 20–50 mg/Nm³, per BCInsight/CRU), which is why acid absorption features in modern designs below.
Wet scrubber configurations and performance
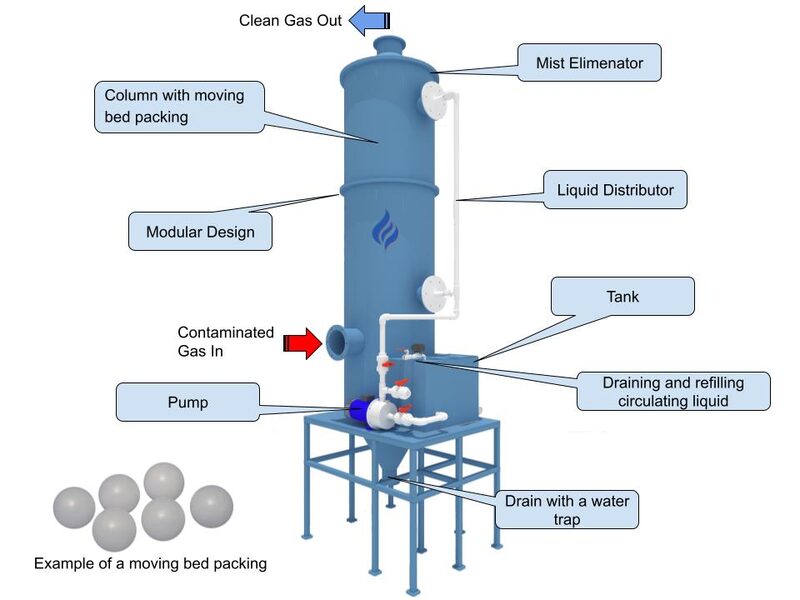
Designers lean on wet scrubbers — packed‑bed (liquid flows over structured or random packing) or Venturi (a high‑velocity throat creates intense droplet‑particle contact) — because prill/granulation air is hot, humid, and contains NH₃. Typical configurations combine multiple stages: quench sprays to saturate and cool, high‑efficiency particle capture, mist elimination, and a dedicated acid stage for ammonia. Stamicarbon’s MMV (MicroMist‑and‑Venturi) system, for instance, installs a quench vessel downstream to saturate incoming gases, followed by venturi tubes, demisters, and an acidic packed stage for residual NH₃ (BCInsight/CRU; BCInsight/CRU).
Single‑stage water scrubbers (packed‑bed “dust scrubbers”) routinely cut dust to ≲30 mg/Nm³ (BCInsight/CRU). The excess fine urea dissolves into the circulating water, yielding about 40–45 wt% urea solution (BCInsight/CRU). Toyo’s water‑packed system runs at only 50–150 mm H₂O pressure drop (BCInsight/CRU), and has been applied worldwide (including Kaltim No.5). Water scrubbing alone does not remove much NH₃ (ammonia is only sparingly soluble without acid).
Injecting mineral acid (H₂SO₄ or HNO₃) into a packed‑bed circuit enables simultaneous NH₃ capture. In one commercial single‑stage acid scrub, NH₃ dropped to under 20 mg/Nm³ while dust remained below 30 mg/Nm³ (BCInsight/CRU). The dissolved NH₃ reacts to form ammonium sulfate or nitrate (e.g., 2NH₃ + H₂SO₄ → (NH₄)₂SO₄; Google Patents). The by‑product is a water‑soluble fertilizer liquor (UAS or UAN) that must be handled separately; this approach generally does not recycle the captured urea back to the urea loop (BCInsight/CRU). Acid feed in such stages typically relies on accurate chemical dosing, where equipment like a dosing pump is used to control addition.
Two‑stage designs decouple dust from ammonia capture. The lower water‑packed stage traps urea dust and recovers a ~45 wt% solution (BCInsight/CRU). An upper acid spray or packed stage then absorbs NH₃, producing two liquor streams: a relatively pure urea solution and an ammonium‑salt solution. In practice (as in a Toyo double‑stage), the urea stream goes to evaporation for reconcentration, while the NH₄‑salt stream is processed separately (BCInsight/CRU; BCInsight/CRU).
Advanced Venturi/multi‑stage scrubbers, like Stamicarbon’s MMV, push particulate capture into the sub‑micron domain. At the 1,000 tpd Dakota Gasification Co. plant, an MMV train — quench plus 28+ Venturi tubes, mist stages, and an acid section — achieved finished‑stack total particulate under 10 mg/Nm³ (EPA test) with zero visible emissions (EPA Method 9) (BCInsight/CRU). It produced two blowdown streams: one concentrated urea solution, the other concentrated (NH₄)₂SO₄ (BCInsight/CRU). Similarly, toy setups report final urea‑containing product with only ~0.2–0.3 wt% ammonium sulfate (AS) derived from scrubbing (BCInsight/CRU), indicating highly effective separation. Operating experience at Dakota Gasification found that minor hardware tweaks (adding Venturi tubes and modifying “chimney hat” seals) reduced NH₄SO₄ carry‑over from 0.28% to only 0.002% of the recovered solution (BCInsight/CRU).
Wet scrubber design metrics
Designers size scrubbers using familiar parameters. Empirical L/G ratios (liquid‑to‑gas, litres of scrubbing liquid per cubic metre of gas) on the order of 2–10 L/m³ are common (Torch-Air). Typical pressure drops are modest — tens to a few hundred mm H₂O — so fan power stays manageable (BCInsight/CRU).
Materials must resist urea/acid corrosion — polypropylene packing or stainless steel are common selections. Demister stages (chevron or mesh pads) and “chimney hat” baffles are widely used to curb acid/mist carry‑over (BCInsight/CRU). Corrosion‑resistant hardware such as 316L stainless steel housings aligns with these material requirements.
In practice, most systems operate at very high capture efficiencies: properly‑designed wet scrubbers can remove >95–99% of fine urea dust and NH₃ (Torch-Air; BCInsight/CRU). EPA guidance referenced in industry sources notes packed‑bed NH₃ scrubbers can remove up to ~99% of ammonia (Torch-Air). In the fertilizer industry, this level of control is needed to meet modern emissions limits and to minimize loss of N.
Treatment and recycling of scrubber liquors
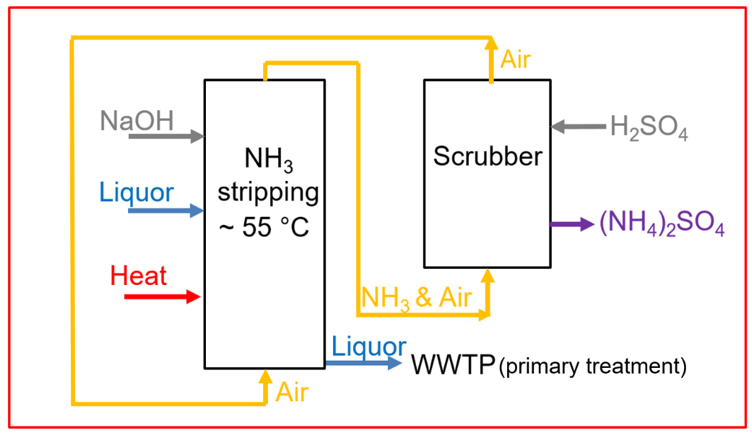
Crucially, scrubber wash water is not waste — it contains valuable nitrogen. In modern designs both streams are recycled.
Urea‑laden water from the dust scrub typically emerges at 30–45 wt% urea. Plants send this liquor to evaporation; a secondary unit concentrates it to ~95+ wt% urea, then pumps it back to the granulator/prilling feed (Google Patents). ThyssenKrupp’s scheme explicitly routes the ~45% urea scrub solution to a second evaporator (decoupled from the main loop) and returns the >95% concentrate to the granulation section (Google Patents). This two‑stage evaporation avoids contaminating the main loop.
The acid‑scrub stage yields an ammonium‑salt solution — typically (NH₄)₂SO₄ or NH₄NO₃ — which is recycled into an acid stream and bled for concentration. It is usually evaporated/crystallized to produce pure ammonium sulfate (or nitrate) fertilizer (BCInsight/CRU). Some licensors allow blending: a mixture of urea and NH₄NO₃ (UAN) can be produced if nitric acid is used (NH₃ + HNO₃ → NH₄NO₃; Google Patents), or urea–ammonium sulfate (UAS) if H₂SO₄ is used. Toyo even offers a “no by‑product” mode: the UAS solution is concentrated in a small evaporator and returned to the urea feed (BCInsight/CRU). In all cases, the key is reusing the N rather than discharging it. By recovering (NH₄)₂SO₄ or UAN, the scrubber water essentially becomes another fertilizer stream.
The result is a dual win: integrated wet‑scrubbing loops clean the air and recycle almost all nitrogen. High‑efficiency packed‑bed or Venturi designs have demonstrated single‑digit mg/Nm³ stack emissions (BCInsight/CRU; BCInsight/CRU), while the captured urea (in water) and ammonia (as NH₄‑salt) are concentrated and fed back, boosting overall N yield — as documented across the cited industry reports and technical literature (BCInsight/CRU; BCInsight/CRU; BCInsight/CRU; BCInsight/CRU).
