In combined-cycle plants, even tens of parts per billion of oxygen can eat an economizer tube. That’s why operators are driving dissolved O₂ to virtually zero and pushing feedwater pH into the high‑9s—with a clear pivot away from hydrazine.
Industry: Power_Generation_(HRSG) | Process: Deaeration_&_Feedwater_Conditioning
Economizer leaks “can occur within hours of oxygen contamination,” one supplier warns—a reminder that thermally deaerated water is only the start of reliable HRSG (heat recovery steam generator) operation (Water Technologies). Even ppt‑level oxygen has been linked to rapid pitting and economizer tube corrosion, so plants chemically “polish” the last traces after mechanical deaeration (Water Technologies) (Dieselship).
A well‑operating deaerator—thermal or vacuum—pulls more than 90–99% of dissolved oxygen via heating/steam stripping, often cutting to single‑digit ppb (≈7–20 ppb) (Power Engineering) (Water Technologies). In practice, operators target under 5–10 ppb dissolved O₂ entering the HRSG to stay ahead of pitting risk. By contrast, some modern plants deliberately use oxygenated treatment (OT)—injecting ~5–150 ppb O₂ along with high pH to form protective iron oxide films—but only with ultra‑pure water and copper‑free metallurgy (Power Engineering) (Power Engineering).
To hit those oxygen targets, plants meter reducers at the condensate or feedwater pump discharge—often via a dedicated dosing pump—and adopt a boiler‑grade program of oxygen scavengers sized to the trace ppb load.
Mechanical deaeration performance
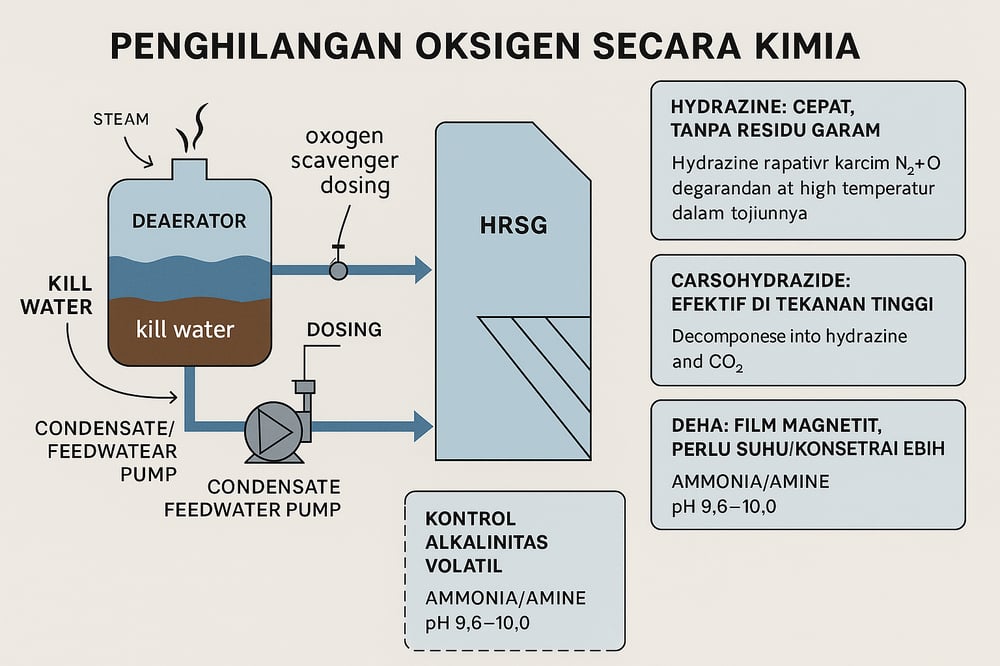
Mechanical deaeration removes the bulk O₂, but “even a 99% mechanical deaerator left a small residual that can still harm the boiler without chemical treatment” (Water Technologies). Targeting feedwater O₂ below 5–10 ppb acts as a safety margin for the economizer, where tens of ppb at high temperature have been tied to rapid tube failure (Water Technologies).
Chemical oxygen scavengers
Once so‑called “coffee‑brown kill water” clears the deaerator, a chemical oxygen scavenger is injected to strip the final ppb before the HRSG. High‑pressure units prefer organic reducers; low‑pressure units sometimes accept inorganic sulfites (Dieselship) (Power Engineering).
Hydrazine (N₂H₄) set the benchmark with a 1:1 stoichiometry to oxygen (N₂H₄ + O₂ → N₂ + 2H₂O), leaving no solids. A rule‑of‑thumb is 1 mg/L hydrazine neutralizes ~1 mg/L O₂, with typical dosing on the order of 0.02–0.15 mg/L (20–150 ppb) in the boiler to ensure feedwater O₂ under 10 ppb (Dieselship) (Power Engineering). Above ~350°C, hydrazine decomposes to NH₃ + N₂, which contributes alkalinity (Dieselship). But this comes with a major caveat: hydrazine is classified in the EU as a C1B carcinogen and is being curtailed or phased out in many regions (Fineamin) (Corrosion (Allen Press)). It is also ineffective at low temperature (<50°C) and, via NH₃ formation, can strip copper oxides and accelerate copper‑alloy corrosion (Dieselship). As a result, many plants are replacing hydrazine with safer alternatives (Fineamin) (Corrosion (Allen Press)).
Carbohydrazide (CHZ, (NHNH)₂CO) is a solid organic scavenger that decomposes in the boiler to hydrazine + CO₂. In practice, plants feed roughly twice the stoichiometric requirement (≈2 moles CHZ per mole O₂) (The Water Network) and maintain feedwater on the order of 0.5–1.0 mg/L (500–1000 ppb) with a boiler residual around ~20–60 ppb for safety margin (ChE Resources) (The Water Network). It is effective at high pressure (system ratings up to ~170 bar) (Dieselship) and leaves no inorganic solids or sulfate. Caveats: CHZ yields NH₃ and CO₂ (the latter can form carbonic acid if carried over, requiring pH control in condensate) and is essentially ineffective below ~9–10 bar—the decomposition often requires ~230°C (The Water Network). Studies show CHZ’s oxygen‑scavenging efficiency comparable to hydrazine under HRSG conditions (Corrosion (Allen Press)), but plants still monitor residuals (via specialty tests) or metal pickup; rising Fe or Cu often triggers incremental feed increases (The Water Network).
Diethylhydroxylamine (DEHA, C₄H₁₁NO) is another organic reducer that forms a thin magnetite film on steels (as hydrazine does) (Dieselship). It reacts more slowly—especially at moderate temperature—often needing higher concentration or higher temperature to match hydrazine/CHZ performance (Corrosion (Allen Press)) (Corrosion (Allen Press)). Byproducts—diethylamine, ethylamine, and acetate when oxygen is present—increase water conductivity but leave no inorganic solids (Corrosion (Allen Press)). DEHA is largely non‑volatile (some fraction can appear in steam), does not accelerate carbon‑steel corrosion, and is popular in Europe and high‑pressure service as a hydrazine replacement (Corrosion (Allen Press)) (Corrosion (Allen Press)).
Other options: At ≤60 bar, sulfite (Na₂SO₃) plus a copper catalyst scavenges rapidly (~8 ppm Na₂SO₃ per 1 ppm O₂), but it adds sulfate solids and depresses alkalinity—so it’s avoided in modern high‑pressure HRSGs. Hydroquinone/erythorbic acid appear in some fossil units or layup, but are rarely needed in combined‑cycle once‑through HRSGs (Dieselship). Where sulfite is used, programs often also emphasize alkalinity control to counter pH depression.
Alkalinity control with AVT chemistry
To suppress acidic attack and flow‑accelerated corrosion (FAC), HRSGs widely use all‑volatile treatment (AVT: volatile amines that raise pH without adding solids). Ammonia (NH₃) is cheap and fully volatile, so many plants dose to hold 25°C feedwater pH at 9.0–10.0 (Power Engineering) (Power Magazine). The equilibrium NH₃ + H₂O ⇌ NH₄⁺ + OH⁻ buffers carbonic acid. In 2013, EPRI raised the recommended range to 9.6–10.0 for all‑ferrous HRSGs, versus older practice (or mixed‑metal systems with copper) at 8.8–9.3 (Power Magazine) (Power Engineering). Raising the LP drum feed pH from ~9.1 to ~9.3–9.5 “practically eliminated further FAC damage” in one case study (WaterTech Online).
pH is logarithmic, so the chemical lift is non‑linear: condensate NH₃ rises from ~2300 ppb at pH 9.6 to ~11,800 ppb at pH 10.0—a roughly fivefold increase to about 2–12 mg/L—and that load can challenge condensate polishers (Power Magazine). Free ammonia is corrosive to copper alloys—“NH₃ is death to copper”—so HRSG circuits avoid copper (Eng‑Tips).
Because of these trade‑offs, many operators inject neutralizing amine blends rather than relying on NH₃ alone. Common volatile amines include cyclohexylamine (CHA), morpholine, methoxypropylamine (MPA), monoethanolamine (MEA), and ethanolamine (ETA); each has distinct volatility and basicity, with MPA tending to remain in LP water and CHA traveling with steam (WaterTech Online) (WaterTech Online) (Power Engineering). A typical approach: feed NH₃ or NH₄OH for bulk pH and add targeted amines—e.g., MPA at the condensate pump for LP circuit pH; small CHA (sometimes morpholine or MEA) in HP feedwater—to achieve liquid‑phase pH ~9.3–9.8 (9.3–9.5 in LP drums is common) (WaterTech Online). When specifying blends, many plants source a neutralizing amine package formulated for their LP/HP steam split.
Monitoring, dosing, and setpoints
All amines and NH₃ are injected at condensate or feed pump discharge, mixed thoroughly, and dosed to pH setpoint—often inferred from conductivity. Because ammonia elevates conductivity, modern practice relies on cation conductivity (CACE) to infer pH; at >9.6 pH, a CACE limit of ≈0.2 μS/cm is recommended (Power Engineering). Instrument panels increasingly monitor pH by differential conductivity after a cation column for sensitivity (Power Engineering). Plants also measure residual amines in condensate occasionally (WaterTech Online).
Typical setpoints in data‑driven programs are: dissolved O₂ <10 ppb; pH(25°C) ~9.6–10.0 (AVT); specific conductivity <0.1 μS/cm or CACE <0.2 μS/cm; and iron <1–2 ppb (Power Engineering) (Power Engineering). Modern technology allows precise control with digital monitors (pH, DO, CACE, FO.), and raising condensate pH from 9.2 to 9.7 has been linked to sharp drops in lifecycle FAC and proportional declines in iron transport (Power Magazine) (WaterTech Online). A well‑run program drives total iron in condensate/feedwater below ~2 ppb (Power Engineering).
There is a trade‑off: higher pH and a positive scavenger residual increase condensate polisher loading. At pH 9.6 to 10.0, NH₄⁺ in condensate can jump from ~2.3 ppm to ~11.8 ppm, saturating ion‑exchange media (especially if run in ammonium form) and raising Na/Cl leakage; silica removal by standard polishers also declines at elevated pH (Power Magazine) (Power Magazine). Many plants respond by operating cation beds in H⁺ form or installing additional polishing so corrosion control does not degrade other purity metrics (Power Magazine) (Power Magazine). Resin performance and leakage behavior become key KPIs; operators often review their ion-exchange resin selection alongside chemistry changes.
Regulatory context and blowdown handling
Indonesia does not specify HRSG feedwater chemistry directly, but boiler blowdown must meet environmental discharge limits. Relevant SNI standards cap blowdown pH at 6–9 and limit ammonia and metals to low ppm or sub‑ppm levels (Global Regulation). Practically, residual NH₃ or amines in blowdown are neutralized before release (often in neutralization tanks). AVT programs help by adding almost no salts, keeping blowdown volumes low.
Market shift away from hydrazine
Globally, hydrazine use is in retreat due to health and environmental rules. German/EU and other Western plants are phasing it out; US OSHA, IARC, and NTP classify hydrazine as carcinogenic (Corrosion (Allen Press)) (Fineamin). In response, modern HRSGs prefer CHZ or DEHA as “non‑hazardous” oxygen scavengers, and many owners proactively adopt alternatives in anticipation of tighter standards (Corrosion (Allen Press)) (Corrosion (Allen Press)) (Fineamin).
Operational takeaway and performance outcomes
A best‑practice HRSG feedwater program looks like this: (1) near‑complete deaeration (DO <10 ppb); (2) continuous scavenger feed to strip residual O₂; (3) AVT chemistry with feedwater pH in the high‑9s to suppress FAC; and (4) continuous monitoring of DO, pH/CACE, iron, and conductivity against numeric targets (Power Engineering) (Power Engineering). Implementing this typically doubles the feedwater ammonia concentration goal (and resin load) compared to older 9.0–9.3 regimes (Power Magazine) (Power Magazine), yet field experience shows it dramatically lowers corrosion rates. Plants adopting the high‑pH/low‑O₂ regime report extended economizer life, fewer leaks, and iron often below 2 ppb (Power Engineering). The balance between chemical usage and polishing burden is real, but data‑driven programs consistently find that “high‑pH/low‑O₂” chemistry prevents most HRSG corrosion problems and supports long‑term availability (Power Magazine).
For execution, the enabling kit is straightforward: injectors sized for trace ppb loads, plus boiler‑grade AVT and scavenger packages. Plants often standardize on a boiler alkalinity control program alongside their oxygen scavenger and amine blends to keep the system within narrow windows season‑to‑season.
