Mixed metallurgy and stray oxygen turn cement plant closed loops into galvanic minefields. The fix, studies show, is a blended inhibitor program that protects copper and steel simultaneously — and tight control of concentration and pH.
Industry: Cement | Process: Closed
In cement plants, closed‑loop cooling systems (sealed recirculating circuits serving heat exchangers and coils) are built from mixed metals: carbon steel, copper, copper‑nickel alloys, and often stainless steel. Oxygen ingress from makeup water or during shutdown can trigger rapid corrosion. One study notes that “dissolved copper…plates onto a steel surface and induces rapid galvanic attack of the steel” (azom.com). The takeaway operators keep repeating: all closed systems must include copper inhibitors, and steel surfaces need anodic passivators such as nitrite or molybdate. The chemistry has to be tailored by metal — and maintained at the right levels and pH.
Laboratory and handbook data converge on the same point. In mixed‑metal coupon tests, a molybdate‑based blend protected both steel and copper alloys better than nitrite alone, which only protected steel (watertechnologies.com). Azole inhibitors by themselves can drive copper alloy corrosion to near zero (researchgate.net), but they do not passivate steel. Modern programs therefore combine azoles for copper with nitrite or molybdate for steel, then hold both concentration and pH within tight control.
Azole protection for copper alloys
Benzotriazole (BTA) and tolyltriazole (TTA) — collectively “azoles” — are the industry standards for copper and brass. They adsorb onto the native cuprous oxide (Cu₂O) on copper alloy surfaces, forming a chemisorbed Cu–azole film that blocks further copper oxidation (watertechnologies.com).
Properly dosed, this film can nearly eliminate copper corrosion. In synthetic cooling water, Admiralty (copper‑nickel) coupons saw corrosion rates as low as 0.04 mpy (mils per year) with an azole inhibitor, versus ~2.7 mpy without (researchgate.net). Facilities typically dose azoles in the mg/L (milligrams per liter) range; a survey notes triazoles are “commonly added in the mg/L range to protect copper” (azom.com). One study found that exceeding about 8–10 mg/L benzotriazole in neutral water led to >80% inhibition of copper (mdpi.com); at very low levels, copper corrodes nearly freely.
Because oxygen and biocides can oxidize the Cu–azole complex, triazoles deplete over time. Industry practice is regular monitoring — ion chromatography (IC) or UV can track BTA/TTA in real time (azom.com) — and testing at least weekly to hold azole above its protective threshold. Underdosing invites Cu leaching, followed by galvanic pitting when copper plates out on steel (azom.com). Modest overdosing is generally safe, though it raises waste and cost.
Nitrite and molybdate passivation on steel
For carbon steel, passivation (forming a stable protective oxide) is essential. Nitrite (NO₂⁻) and molybdate (MoO₄²⁻) are anodic inhibitors for ferrous metals that push the metal potential nobler and precipitate a stable Fe oxide film. Mechanistically, nitrite oxidizes Fe²⁺ to Fe³⁺, forming hematite/goethite that passivates the surface (mdpi.com). Molybdate similarly promotes Fe₂O₃ films and is especially stable in hot or oxygenated water (watertechnologies.com, watertechnologies.com). Chromates are now banned for environmental reasons (watertechnologies.com).
Control limits are quantitative. In steel‑only loops, 600–1200 ppm NO₂⁻ (as NaNO₂) with pH >7 provides strong protection (watertechnologies.com). When steel is coupled to copper alloys, nitrite demand jumps: 5000–7000 ppm NO₂⁻, and up to 10,000 ppm if aluminum is also present (watertechnologies.com). In chloride‑containing water, raising nitrite from 7000 to 9000 ppm increased inhibition from ~72% to ~97% (mdpi.com). A molybdate‑based program instead typically holds 200–300 ppm MoO₄²⁻ to cover steel and steel/Cu systems (watertechnologies.com). Molybdates are effective in “hard” or hot waters, but cost more.
Concentration control and microbial interactions
Closed loops lack blowdown losses, yet inhibitors are consumed by side reactions and oxygen ingress. Biological activity can reduce nitrite, so occasional biocide shock or bleed may be needed (watertechnologies.com). For such interventions, many contractors rely on biocides designed to control biofilm.
Operators monitor inhibitor levels chemically: simple test strips or ferrous‑chelate colorimetry for nitrite, and UV or IC for azoles. Industry “control limits” matter — one reference labels 3000 ppm nitrite (with pH ~10) as a lower alarm level (mdpi.com). If nitrite falls short (<2000 ppm in mixed‑metal systems), steel can corrode at several mil per year. Regular sampling and make‑up dosing keep programs within spec; accurate chemical dosing helps hold tight setpoints, a task commonly handled with a dedicated dosing pump.
pH window and aluminum constraints
Inhibitor effectiveness depends strongly on pH. For steel passivation, aim for pH ~8.0–8.5. Veolia guidance notes nitrite programs require pH above 7.0 — ideally 8–9 — to sustain the oxide film (watertechnologies.com). Below ~7, the passive film dissolves and hydrogen evolution accelerates. Extremely high pH can precipitate scale or attack aluminum, though cement systems seldom use aluminum alloys.
For molybdate programs, hold pH 7–9; listed recommendations are 200–300 ppm MoO₄²⁻ at pH 7–9 (watertechnologies.com). If aluminum components are present (rare in cement plants), pH should not exceed ~9 (watertechnologies.com). Many water chemists adjust weekly with dilute acid or base; acid feed is often required if high nitrite doses push pH above the target range (watertechnologies.com).
Performance targets and observed results
With correct chemistry, corrosion can be driven very low. Common targets are ≤0.1 mm/year (4 mpy) on steel and <0.01 mm/year on copper. Literature shows near‑100% inhibition is attainable: ~97% reduction on steel at ~9000 ppm nitrite (mdpi.com) and essentially full protection of copper alloys with ~10 ppm TTA (mdpi.com). In mixed‑metal coupon studies, adding a molybdate/nitrite/azole blend reduced both steel and copper corrosion to negligible levels, whereas without such control coupons corroded orders of magnitude faster.
One lab test reported steel coupons in a 100 ppm‑chloride system at 0.0014 mm/year (0.055 mpy) when properly inhibited — roughly 100× lower than the typical industrial threshold of 0.125 mm/year cited by standards. Multi‑component programs are the throughline; where appropriate, contractors turn to blended closed‑loop chemicals to simplify treatment.
Contractor and maintenance manager checklist
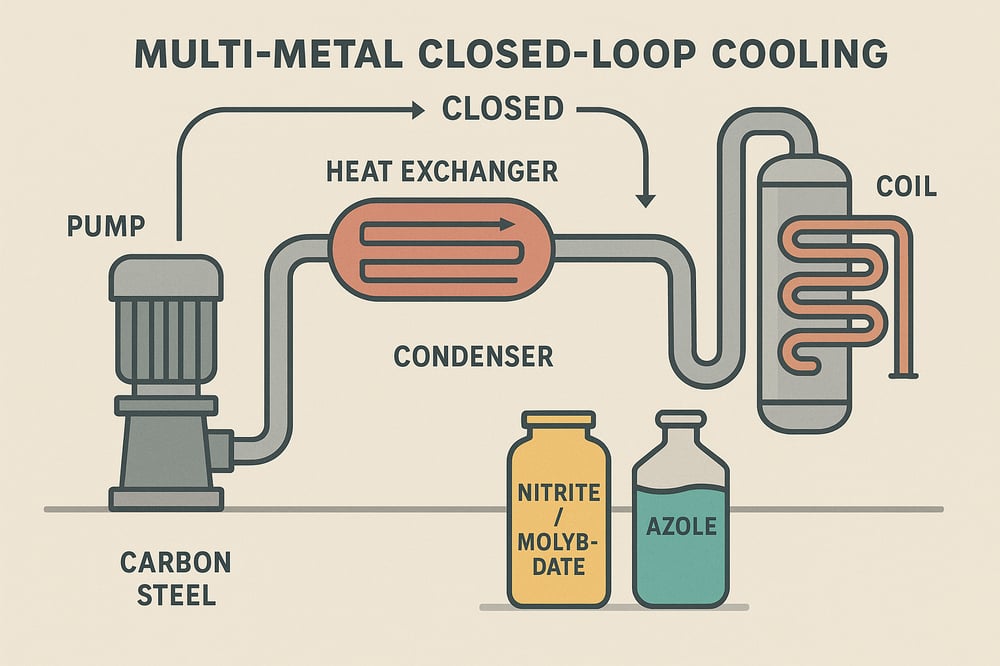
Use both inhibitor classes. Always include a benzotriazole or tolyltriazole to protect any Cu/CuNi parts, typically in the 5–20 ppm range (azom.com). Dose nitrite or molybdate separately for steel. In practice, a blended product containing nitrite + low‑level molybdate + azole is often used to simplify monitoring. One custom formulation (60 ppm MoO₄ + 20 ppm NO₂ + 20 ppm organic + 10 ppm Zn) delivered steel protection equivalent to 1000 ppm MoO₄ alone (onlinelibrary.wiley.com). For sourcing, many providers group such blends as corrosion inhibitors for closed circulation.
Maintain inhibitors above critical thresholds. As a rule of thumb: at least 1000–1500 ppm NO₂⁻ for steel‑only loops, and ≥5000–7000 ppm where copper alloys are present (watertechnologies.com). Alternatively, 200–300 ppm MoO₄²⁻ can serve as the main passivator (watertechnologies.com). Ensure benzotriazole remains in the single‑digit to low‑double‑digit ppm so copper stays fully coated; ≥8 ppm yields strong inhibition (mdpi.com). Measure and log each inhibitor at least weekly. Many operators use online analyzers or beaker tests to verify mg/L of nitrite and azole.
Control pH carefully. Set bulk water around pH 8.0–8.5 (ideally 8.2), monitor daily, and adjust with acid or base to counter drift. After nitrite additions — which tend to raise pH — bring it back toward the target. Maintaining the pH window keeps passive films intact. Tightly run programs benefit from metered additions delivered by an accurate dosing pump.
Inspect and test efficacy. Install periodic corrosion coupons or electrical probes in representative loops. Many plants aim for <0.1 mm/year on steel. If rates approach this limit, tighten inhibitor control or re‑check pH. If accelerated corrosion is observed (e.g., ≥1 mpy), suspect inadequate inhibitor or off‑spec pH. First signs of under‑inhibition in mixed systems often include copper staining or incipient pitting on steel; watch water copper content and any reddish rust deposits.
Safety and environment. Nitrate/nitrite and molybdate have environmental limits. Closed‑loop systems discharge minimally, but any blowdown must meet local regulations. In Indonesia, industrial wastewater standards generally limit nitrate levels (for example, <50 mg/L NO₃–N in Indonesian «PP82/2001 KBLI» for many industries), so ensure blowdown is very dilute or treated. Azoles are typically organic and biodegradable; copper corrosion inhibitors usually remain in the circuit and are not discharged if the loop is sealed. Keep safety data current and use protective gear when handling concentrated inhibitors.
Bottom line and sources
The throughline from handbooks and studies is consistent: a balanced regimen tailored to mixed metallurgy, with azoles for copper, nitrite or molybdate for steel, and tight pH control around 8–9, minimizes metal loss and avoids unplanned shutdowns. Veolia’s handbook notes that nitrite/molybdate/azole blends “provide the best corrosion protection [for mixed] metallurgy” (watertechnologies.com), and field studies confirm that proper dosing can suppress corrosion by well over 90% (researchgate.net, mdpi.com). The operational playbook above is supported throughout by industry handbooks and peer‑reviewed work: watertechnologies.com; watertechnologies.com; watertechnologies.com; onlinelibrary.wiley.com; mdpi.com.
