At 50–100 bar, a few parts per billion of oxygen or carbon dioxide can eat through steel. High‑pressure ammonia/urea plants are turning to a tight mix of deaeration, DEHA oxygen scavenging, and volatile amines—watched by continuous analyzers—to keep dissolved oxygen at ≲5–10 ppb and condensate pH in the safe zone.
Industry: Fertilizer_(Ammonia_&_Urea) | Process: Boiler_Feedwater_&_Steam_Generation
High‑pressure boilers in ammonia/urea fertilizer plants demand ultra‑pure, oxygen‑ and CO₂‑free feedwater, with dissolved oxygen (DO) held to ≲0.005–0.01 mg/L (5–10 ppb) and iron to ≲0.005–0.01 mg/L (ro.scribd.com) (ro.scribd.com). Silica is kept <5 ppm and chlorides <50 ppm for high‑pressure service (www.boilermart.co.id). Alkalinity is run low (P‑ and M‑alkalinity ~20–50 ppm) and feed pH ≈8.5–10—adjustable to 8.8–9.2 at 250 °C (ro.scribd.com) (ro.scribd.com). Good purification enables high cycles of concentration (often TDS <1000 mg/L) (www.boilermart.co.id) (www.xylem.com). Indonesian boiler practice mirrors these values and recent regulations (Permen 11/2023) emphasize efficiency and clean processes in the ammonia/urea industry (www.boilermart.co.id) (id.scribd.com).
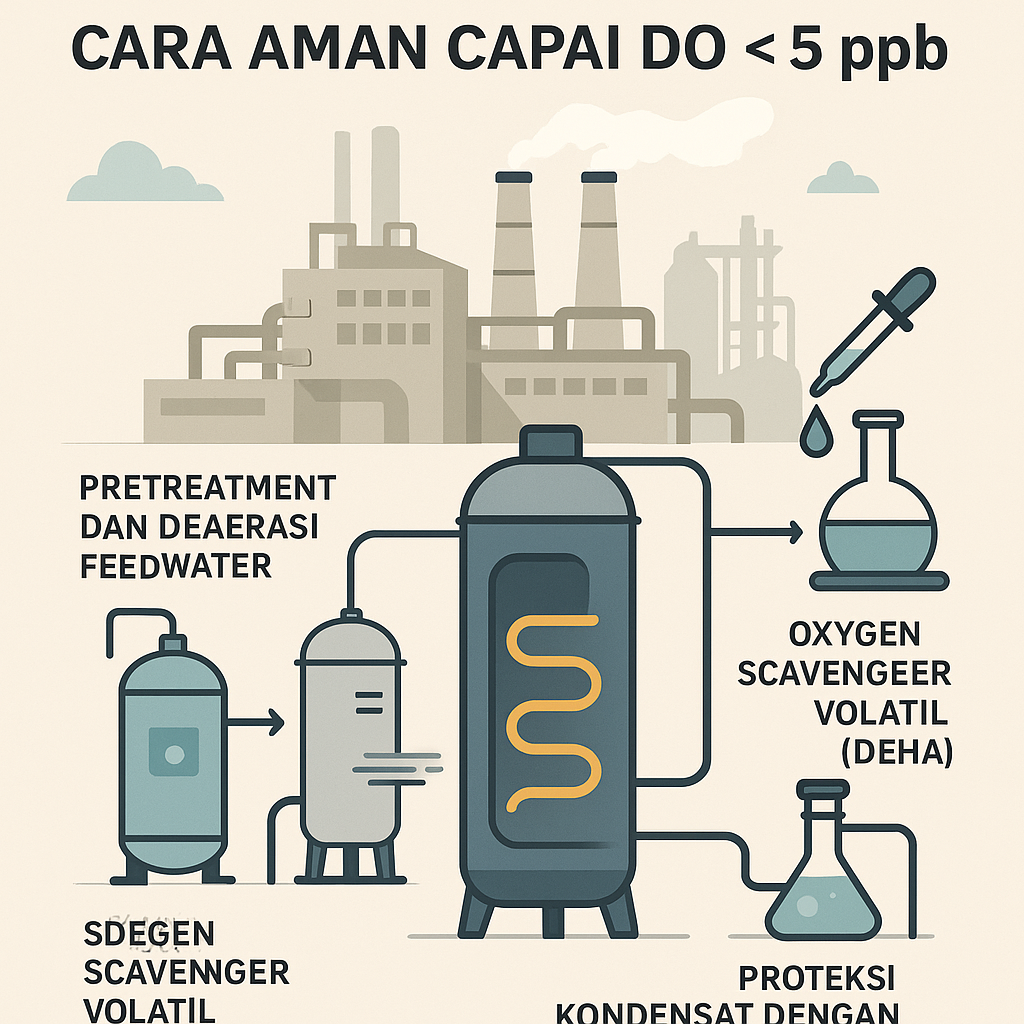
The strategy is straightforward: remove hardness and alkalinity up front; mechanically strip oxygen and free CO₂; then chemically maintain safe pH and residuals in the boiler and the condensate return. As Xylem puts it, investing in equipment that produces higher‑quality boiler feedwater reduces chemical usage and increases cycles of concentration, improving overall plant efficiency (www.xylem.com).
Feedwater pretreatment and deaeration
Pressure deaerators (spray‑tray units) heat makeup water close to boiling, releasing ~97–98% of dissolved oxygen and free CO₂ (www.watertechnologies.com) (www.watertechnologies.com). With good operation, vendors guarantee DO ≲0.005 cm³/L (~7 ppb) (www.watertechnologies.com). For perspective, at 90 °C ordinary water still holds ~2 ppm O₂, so continuous steam venting is needed to approach zero (www.forbesmarshall.com). Mechanical deaeration removes most free CO₂, but combined CO₂ from bicarbonate alkalinity remains and later flashes into steam, condensing as carbonic acid—hence the need for downstream chemical steps.
Upstream, a softener/dealkalizer removes hardness and alkalinity (cutting the CO₂ load). Installing a dealkalization unit after hardness softeners “reduce[s] the alkalinity of the makeup water going to the boiler,” slashing both carbonic acid formation and neutralization demand (www.chemaqua.com). Plants commonly deploy reverse osmosis (RO) or ion exchange polishing; RO routinely removes ~99% of dissolved minerals (www.xylem.com). In practice, a softener can be paired with a brackish-water RO, then an ion‑exchange demineralizer using ion exchange resins for polishing. Some plants dose sodium bisulfite (Na₂SO₃) in the feedwater tank as a polishing oxygen scavenger, but only for low‑pressure boilers because it adds solids and is not used for very high‑pressure or high‑purity units.
After pretreatment and deaeration, operators check DO and CO₂. Feedwater temperature at 85–95 °C ideally lowers DO to <5–10 ppb (www.galgo.co.uk). Monitoring is continuous or frequent: a trace‑DO analyzer or portable Rhodazine‑D kits (ASTM D5543) measure down to ~2 ppb (www.galgo.co.uk), with samples collected to avoid air contact (www.galgo.co.uk). In parallel, feed pH/TDS are measured; operators target feed pH ≈8.5–9.0 to optimize oxygen scavenging kinetics (ro.scribd.com) (www.watertechnologies.com).
Oxygen scavengers (DEHA) and passivation
Residual oxygen after deaeration can trigger economizer and boiler corrosion; pitting rates double about every ~10 °C rise (www.forbesmarshall.com), and rapid localized failures have been documented within hours of oxygen ingress . High‑pressure units (>60 bar) steer clear of inorganic sulfites (steam contamination) and hydrazine (toxicity), favoring organic, volatile scavengers. Diethylhydroxylamine (DEHA) is widely used: it is volatile (distributes with steam), strongly scavenges O₂, and passivates steel to magnetite (ro.scribd.com) (ro.scribd.com). In a boiler program, this sits squarely under oxygen scavengers.
DEHA reacts with dissolved O₂ to yield nitrogen, water, and a small amount of acetic acid (ro.scribd.com). Stoichiometrically, 1 mg/L O₂ consumes ~1.2 mg/L DEHA, but plants commonly use a 3:1 ratio to hold excess; for example, removing 0.5 ppm DO would require ~1.5 mg/L DEHA (ro.scribd.com). A typical program aims for 80–120 mg/L DEHA residual in condensate; Kasinecz (2001) reports that 300–500 mg/L initial feed will build ~80–120 mg/L in condensate, whereas very high‑pressure units may require only ~75–100 mg/L feed (trace residual ~40–50 mg/L) (ro.scribd.com) (ro.scribd.com). Because DEHA is tricky to assay directly, operators reference colorimetric PDTS or cupric CUPD methods to infer concentration (www.galgo.co.uk).
Application matters. Best practice is to inject into the deaerator storage leg or feedwater line upstream of any bleed‑off, not at the deaerator dome where it would vent (ro.scribd.com). Avoid co‑feeding with sodium sulfite at the same point (DEHA will decompose it) (ro.scribd.com). Metering is done with a dosing pump, typically flow‑proportional or tied to boiler resets. Effectiveness is tracked by dissolved oxygen and iron: DO ≲0.005 mg/L, and total iron in feedwater and condensate below ~0.01 mg/L (ro.scribd.com) (ro.scribd.com). Plants also use brief DEHA “off” checks to verify DO starts to rise. Quantitatively, well‑treated systems see negligible iron and DO; one case showed condensate iron <0.005 mg/L under DEHA versus >0.05 mg/L unprotected. Because DEHA is volatile and passivating, Kasinecz notes it can maintain condensate pH by degrading to trace diethylamine/ethylmethylamine—sometimes eliminating the need for separate neutralizing amine (ro.scribd.com) (ro.scribd.com), though many sites still feed a neutralizing amine for CO₂ control.
Condensate return protection (amines)
Because condensate is ultra‑pure, tiny CO₂ loads from feed bicarbonates form carbonic acid and drop pH, causing the classic “grooving” of return lines and traps (www.chemaqua.com). Two approaches are common. Neutralizing amines—volatile bases like morpholine, cyclohexylamine, and diethylaminoethanol (DEAE)—vaporize with steam and neutralize carbonic acid on condensing, targeting condensate pH ≈9–10. Typical programs hold 5–15 mg/L amine residual or condensate pH ~9.5, with checks at various return points to ensure pH never falls below ~8.5–9 (ro.scribd.com) (www.chemaqua.com). This is typically implemented with a neutralizing amine feed.
Film‑forming amines (e.g., octadecylamine or proprietary blends) deposit a hydrophobic barrier that blocks both CO₂ and O₂ attack; with films, condensate pH is often run around 6–7 (www.chemaqua.com). Because films form gradually and can mobilize deposits, start‑up dosing is staged (e.g., ~⅓ of normal initially) for better distribution (www.chemaqua.com). Protection is verified via spool‑piece or coupon tests; a well‑coated surface will bead condensate like wax on a car (www.chemaqua.com). Many plants combine a neutralizing amine with a low‑dose film‑former, or pair DEHA with morpholine. As Chem‑Aqua notes, “The most common method of preventing carbonic acid attack is through neutralizing amines… Filming amines provide a protective barrier against both carbonic acid and oxygen” (www.chemaqua.com).
Key metrics are condensate pH and iron. Morpholine‑style programs hold pH ≈9–10 (film‑programs ≈6–7), with sampling at the boiler inlet, mid‑return, and other points to ensure pH never drops below ~8 in an AVT (all‑volatile treatment) program. Total iron should remain <0.01 mg/L, often undetectable. If low pH or high iron appears, operators increase amine feed and check for air in‑leaks.
Chemical dosing control and monitoring
Dissolved oxygen: a high‑sensitivity analyzer (galvanic, polarographic, or optical) sits on the deaerated feed line to hold ≤0.005 mg/L. Portable Rhodazine‑D kits (ASTM D5543) verify readings at least weekly (www.galgo.co.uk). Trend DO and DEHA feed; if DO exceeds 0.01 mg/L, DEHA rate is increased until it drops. These analyzers, part of the plant’s water treatment ancillaries, also flag deaerator upsets or air in‑leaks.
Oxygen scavenger residual: DEHA is tracked with reference kits (PDTS colorimetric); a small residual in condensate (e.g., 40–80 mg/L, depending on system) is targeted, and once a stable magnetite film exists, 10–50 mg/L may suffice (ro.scribd.com). Conductivity/TDS: boiler solids are controlled by blowdown to design limits (often ~8–10 cycles). Excess sulfite or phosphate shows up as high TDS; too much sodium bisulfite raises TDS and forces extra blowdown (www.forbesmarshall.com).
pH: continuous probes in the drum and condensate (at pump suction or feedtank) target boiler pH ~9.5–10 under AVT, with condensate per the chosen program. Low pH alarms (<8.5 in condensate or <9.0 in boiler) trigger corrective action. Corrosion control: coupons or spool‑pieces in return and feed lines are examined monthly; with film‑formers, beading is assessed (www.chemaqua.com). Lab tests: total iron by ferrozine or AAS is kept <0.01 ppm; DO is checked after the deaerator; neutralizing amine residuals are titrated (often 10–50 mg/L as base). Data and automation: DCS/SCADA logs readings and controls pumps—DEHA pump speed can be linked to feedwater flow or a DO controller, with alarms on DO, boiler level, and condensate pH.
Where a phosphate/polymer program is retained for hardness hold, it is integrated as scale control alongside the all‑volatile chemistry, and dosing is metered with the same dosing pump logic used for DEHA and amines.
Measured outcomes and operating benchmarks
Corrosion control: economizers and headers remain free of oxygen pitting; condenser piping stays pristine. “Less than 0.007 ppm [O₂] is considered practically oxygen‑free” (ro.scribd.com), and the program aims even below that. Iron and copper pickup drop to <0.01 ppm (ro.scribd.com), often to undetectable levels.
Chemical usage: higher‑quality feed plus AVT can reduce chemical costs; replacing a phosphate‑scalant‑heavy program with all‑volatile treatment reduced solids carryover and let cycles rise from ~5 to ~8. As industry guidance notes, higher‑quality feedwater can cut chemical dosing and blowdown losses (www.xylem.com).
Reliability: with DO and CO₂ curbed, unplanned tube‑leak shutdowns decline. Case studies show DEHA + filming amine programs “reduced maintenance and downtime while increasing efficiency” (www.watertechnologies.com) (www.watertechnologies.com). Energy: every gallon of condensate reused saves ~1 cubic foot of natural gas, and stable condensate chemistry allows 90–95% condensate return (www.chemaqua.com).
Complete treatment program summary
The high‑pressure ammonia/urea boiler program includes: thorough pretreatment (softening, dealkalizer/RO, degasser) to minimize impurities; precise boiler conditioning—polymer/phosphate scale control if retained, plus organic oxygen scavengers like DEHA at ~0.05–0.1 g/L feed to achieve ~0.01 g/L condensate residual (ro.scribd.com)—and volatile amine protection (typically a neutralizing amine to hold condensate pH ≈9–10, optionally supplemented by a film‑former). Dosing is governed by continuous monitoring: feedwater DO sensors, boiler/condensate pH meters, iron analyses, and corrosion coupons inform adjustments. By targeting DO <5 ppb and condensate pH per design, plants achieve data‑verified corrosion protection that aligns with international practice and Indonesia’s green‑industry standards (www.boilermart.co.id) (id.scribd.com). For membrane and IX trains, plants routinely lean on membrane systems in front of RO and IX polishing media before the boiler.
Sources used above include boiler‑water handbooks and chemical application notes. Veolia’s Water Handbook explains deaeration and corrosion mechanisms (www.watertechnologies.com) (www.watertechnologies.com); Galgo covers DEHA testing (www.galgo.co.uk) and DO monitoring (www.galgo.co.uk) (www.galgo.co.uk); Chem‑Aqua details condensate corrosion control (www.chemaqua.com) (www.chemaqua.com); and Kasinecz provides DEHA dosing and distribution data (ro.scribd.com) (ro.scribd.com).
