Minor elements in raw meal and fuels don’t just pass through a cement kiln — they recirculate, concentrate, and cause costly buildups. A targeted gas bypass that bleeds off 1–10% of kiln exhaust is proving to be the pragmatic control knob.
Industry: Cement | Process: Preheating_&_Calcination
In a modern dry‑kiln line, the real drama begins before the rotary kiln. Raw meal is successively heated in cyclone preheaters (multi‑stage gas‑solids heat exchangers) and an inline calciner (a separate combustion chamber) before the mix enters the kiln at about 1450 °C. Most of the heat — often 60–70% of total thermal energy — is supplied in the preheater/calciner, where endothermic calcination (CaCO₃ → CaO + CO₂) starts the clock on clinker mineral formation.
Under these counter‑current conditions, trace compounds — alkalis (Na₂O + K₂O), sulfur (as SO₃), and chlorine — volatilize at high temperature and then re‑condense on cooler surfaces. As Enders and Haeseli put it, “thermally unstable compounds like alkali chlorides, alkali sulfates and Ca‑sulfates” vaporize in the burning zone and are carried with flue gas to condense in the preheater (ResearchGate). Chlorine volatilization around 900–1200 °C in the sintering zone and condensation in the riser pipes and lower cyclones at roughly 700–900 °C drive low‑melting salt mixtures (KCl/NaCl/K₂SO₄) to stick to cyclone walls and ducts (ResearchGate).
Because raw materials and alternative fuels (plastics, biomass, industrial wastes) often contain 0.05–1% Cl and similar alkalis, each tonne of clinker can carry hundreds of grams of these volatiles. Solid recovered fuel (SRF) averages about 0.7–0.8% Cl by weight, yet only a small fraction — 0.7–13% — ultimately fixes into clinker, leaving most to recirculate or exit with dust (ResearchGate; ResearchGate).
Volatile cycles and plug risks
The result is a classic “alkali‑chloride cycle”: repeated evaporation and condensation steadily enriches chlorides, alkalis, and sulfates in the preheater–kiln loop. The European Cement Research Academy (ECRA) notes that greater use of alternative fuels has “led to higher inputs” of these volatiles, which become “enriched through circulation between the sintering zone and the lowermost cyclone stage” (ECRA).
Deposits show up where they hurt most: coatings and rings in kiln inlet ducts and low‑cyclone ducts, with hard rings near the calciner or burner level. The operational symptoms are familiar — rising preheater pressure drop, plugged calciner notches, erratic feeder behavior, and the recurring need to quench or mechanically break rings. Enders & Haeseli underscore that volatilization–condensation cycling “reduces the stability of the kiln process,” causing unintended stops and extra maintenance (ResearchGate).
Practical limits are tighter than many expect. Indonesian industry guidance warns that total chloride input above about 0.05% (loss‑on‑ignition‑free basis) typically drives severe plugging; keeping Cl below roughly 0.02–0.03% is “normal” (Scribd). In a cyclone/precalciner kiln without a bypass, operators generally limit Cl input to about 200–300 mg per kg clinker; exceed that and blockage risk rises quickly (Scribd). Excessive alkali/sulfur can also complex with devolatilizing CaO to form sticky sulfates (CaSO₄) or alkali‑rich silicates, worsening buildups.
Gas bypass design and placement
A proven countermeasure is the kiln gas bypass: diverting a portion of the hot kiln exhaust through a separate cooling and de‑dusting loop to prevent recirculation. Plants bleed 1–10% of the gas, often from the kiln inlet or lower riser where volatile chloride concentration peaks and entrained dust is minimal, and where temperature is roughly 650–800 °C (ECRA). ECRA calls the gas bypass “particularly effective for the limitation of chloride cycles” (ECRA), and computational flow modeling is often used to pinpoint the optimal tap location.
Quantitatively, well‑designed systems remove about 20–75% of total chloride input according to ECRA surveys (ECRA). In the field, operators commonly run roughly 5–15% gas flow bypass; FLSmidth/IPPC data cite a 10–15% bypass as “typical” for a chlorine bypass (pdfcoffee.com). A rule of thumb used in practice: each 1% of bypass flow allows an extra ~100 mg Cl/kg clinker to be tolerated, so an 8% bypass would lift the tolerance up to ~800 mg/kg (versus ~200–300 mg/kg without a bypass) (Scribd).
Sulfur and SO₂ co‑benefits
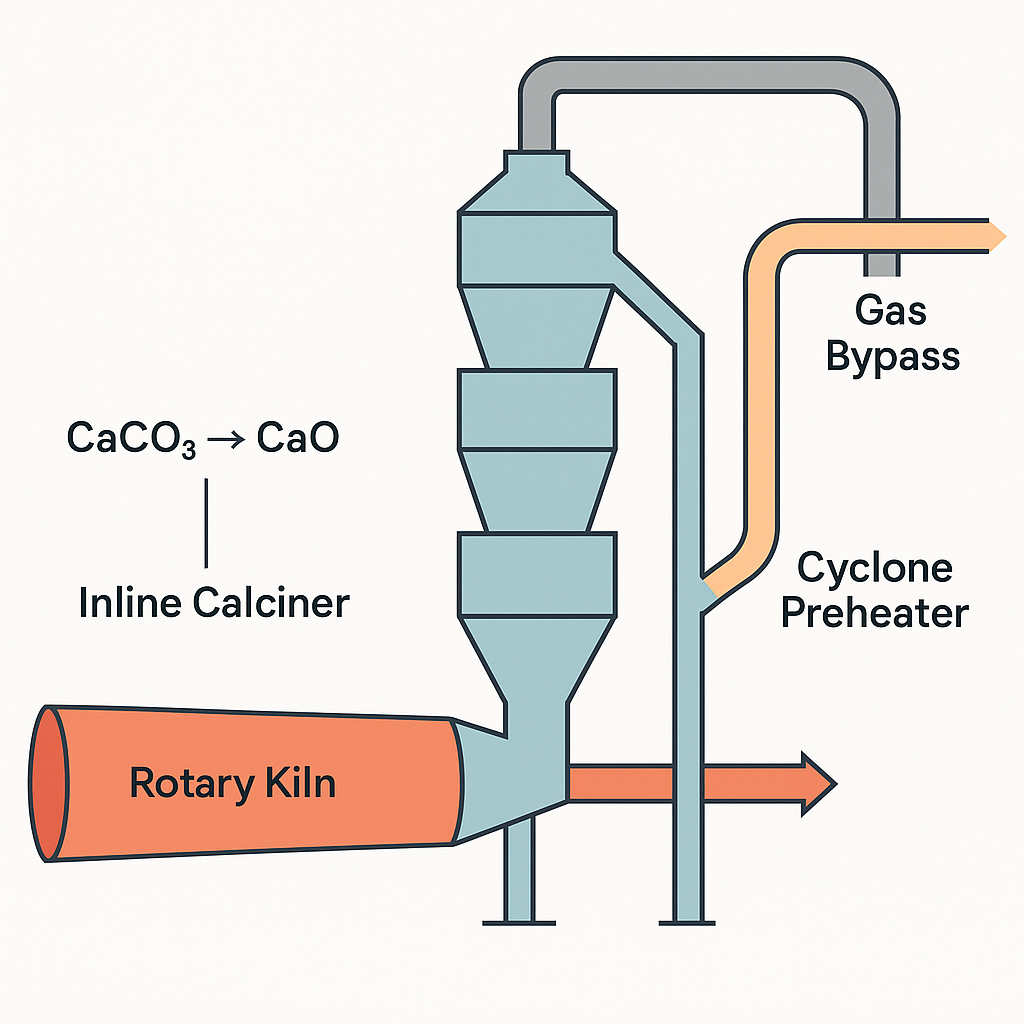
Such partial recirculation also scrubs sulfur. Some SO₂ and alkali sulfates leave with the bleed and are captured — by wet scrubbers or baghouses on the bypass line — though removal is modest: about 2–12% of total sulfur input in surveys (ECRA). By comparison, alkali sulfates, which bind to dust, largely remain; typical chloride removal via bypass far exceeds that for SO₃.
Bypass designs frequently integrate Ca(OH)₂ or slurry scrubbers at the kiln hood to convert SO₂ in the bleed gas to gypsum, limiting stack SOₓ. Patents and Taiheiyo Cement designs use CaO/Ca(OH)₂ in the bleed circuit for SO₂ capture, often also recovering chloride salts in the slurry (ECRA). Plants that meter lime slurry into these scrubbers tend to pair them with accurate chemical feed gear such as a dosing pump for stable reagent ratios.
Bypass dust handling and reuse
Filtering the extracted bleed stream produces a high‑chloride “bypass dust” or slurry that is far richer in volatiles than ordinary kiln dust. Academic tests show bypass dust with several‑fold higher Cl and K; air‑classification can yield a fine fraction 2–3× higher in Cl than the feed, which companies sometimes leach to recover potash (ResearchGate; ResearchGate). Because of the salt load, dust is often landfilled or used in niche applications; only a small, controlled portion typically returns to the kiln as an additive to keep alkali levels in balance.
Economics and fuel flexibility
Despite added handling, bypass systems can pay for themselves by preventing downtime and opening the door to higher‑chlorine waste fuels. A case in point: Votorantim Cimentos in Brazil installed a US$10 million chlorine bypass on a 5 Mt/yr line, lifting co‑processing from 45 kt/yr to 73 kt/yr (+62%), replacing 12 kt/yr of petcoke and cutting ~12,200 t/yr CO₂ (about 5%) (CemFuels).
In sum, a targeted kiln‑gas bypass acts as a purge for volatile alkali and chloride species — often achieving greater than 20% total Cl removal in surveys (ECRA) — stabilizing preheater chemistry, curbing buildups, and enabling more aggressive alternative‑fuel strategies.
Sources and temperature windows
Key studies and industry sources on alkali/chloride cycles and bypass performance: ECRA; ResearchGate; ResearchGate; ECRA; ECRA; ResearchGate; Scribd; Scribd; pdfcoffee.com; CemFuels. Temperature windows for chlorine volatilization (about 900–1200 °C) and condensation (about 700–900 °C) are detailed in Enders & Haeseli (ResearchGate).
