Raw AMD can hit pH≈2.5 with Fe≈46 mg/L, Al≈86 mg/L, and Ni≈0.75 mg/L. Hitting Indonesia’s Ni ≤0.5 mg/L at pH 6–9 takes a stepwise alkaline playbook—lime for bulk neutralization, caustic for fine control—and a final nickel polish.
Industry: Nickel_Mining | Process: Acid_Mine_Drainage_(AMD)_Treatment
Nickel mines that tap sulphide ores leave a stubborn legacy: acid mine drainage (AMD), an acidic effluent that mobilizes metals. One pilot-scale study logged raw AMD around pH≈2.5 with iron ≈46 mg/L, aluminium ≈86 mg/L and nickel ≈0.75 mg/L (onlinelibrary.wiley.com). Indonesia’s discharge bar for nickel mining is tight—Ni below 0.5 mg/L with pH 6–9 (nikel.co.id; the same table references “maksimum Co: 0.4 mg/L”).
The route to compliance isn’t one-and-done. It’s active, multi-stage neutralization with selective precipitation, then a polishing step to catch the last nickel. pH (a measure of acidity/alkalinity) is the steering wheel; hydroxide “flocs” (metal hydroxide particles with high surface area) are the workhorses.
Alkaline reagent economics and kinetics
Hydrated lime (Ca(OH)₂) is the workhorse neutralant. It forms fine flocs that sorb metals and buffers pH strongly. Caustic soda (NaOH) is faster-reacting but is commonly 2–3× more expensive per unit alkalinity (onemine.org). Limestone (CaCO₃) is cheapest but dissolves slowly and often “armors,” stalling removal.
Surface area matters. Neutralization with NaOH or Ca(OH)₂ produced precipitates with BET surface areas ~170 m²/g—an order of magnitude higher than CaCO₃ at ~17 m²/g (onlinelibrary.wiley.com). At pH≈6.5, the high‑surface flocs captured heavy metals, while CaCO₃‑treated water showed negligible Ni uptake (onlinelibrary.wiley.com). In one test, neutralizing to pH 6.5 left Ni ≈0.32 mg/L with NaOH and ≈0.41 mg/L with Ca(OH)₂—roughly 60% lower than with limestone, which removed no Ni (onlinelibrary.wiley.com).
Operationally, Ca(OH)₂ is widely available and is especially cost‑effective for large flows/high acidity (onemine.org). NaOH can be held in reserve for rapid fine adjustment or emergencies. Quicklime (CaO) is viable at scale but needs slaking gear; this design assumes slaked lime. Soda ash (Na₂CO₃), dolomite, or magnesia have niche roles (e.g., EBOR systems). For dosing control, plants typically meter reagents with a dosing pump tied to pH feedback.
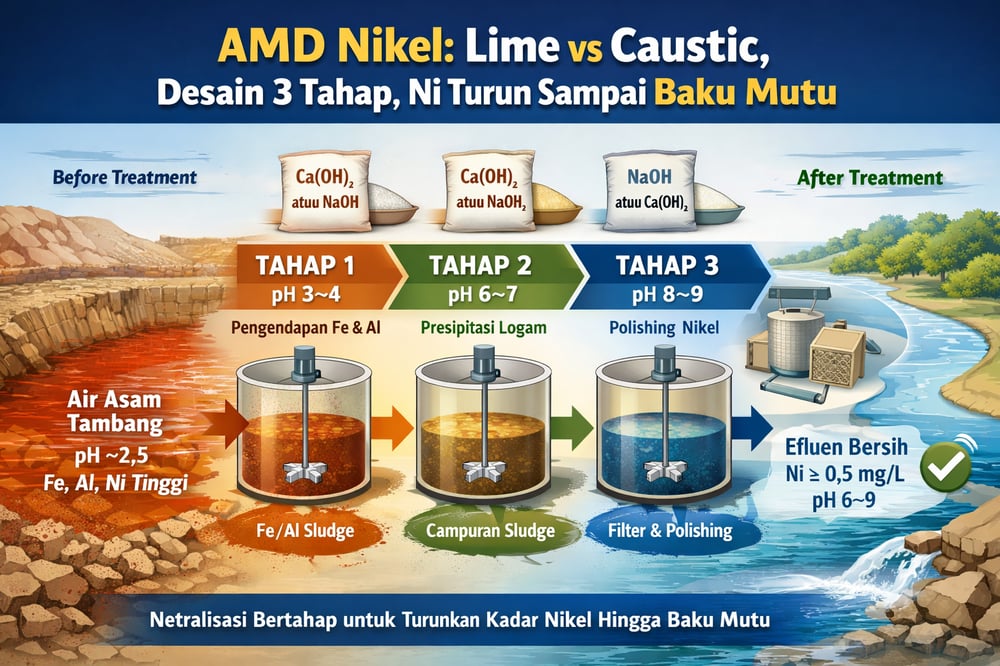
Stage 1: Fe/Al drop‑out at pH 3–5
Start by oxidizing ferrous iron (Fe²⁺) to ferric (Fe³⁺) via aeration or a chemical oxidant. Raise pH into the 3–4 band with lime or caustic. Ferric iron hydrolyzes and precipitates effectively by pH ~3.3, creating an observable buffer zone near pH 3–3.5 (onlinelibrary.wiley.com). In practice, taking industrial AMD to pH≈4 removes >99% of total Fe and generates abundant Fe/Al hydroxide floc; amorphous Al(OH)₃ begins precipitating by pH ~4–4.5, with one study observing near‑complete Al removal just above pH 4 (onlinelibrary.wiley.com).
Design for 20–30 minutes of mixing/settling. The Fe/Al‑rich sludge (on the order of tens of % by weight) offers surface sites for adsorbing other metals; Ca(OH)₂‑treated AMD yielded sludge ~30 wt% Fe/Al oxides in one case (onlinelibrary.wiley.com). Note: Fe/Al removal liberates protons, consuming alkalinity. Settling is typically handled in a clarifier.
Stage 2: neutral pH for mixed metals
After Stage 1 clarification, the pH ~4–5 supernatant still carries heavy metals. Raise pH to ≈6–7. Residual Al continues dropping through pH ~5–6. Transition metals begin coprecipitating or adsorbing onto Fe/Al flocs: bench tests saw Zn and Cu coprecipitating as pH passed ~6.0, and Ni starting to incorporate by pH≈6.5 (researchgate.net; onlinelibrary.wiley.com). In a synthetic laterite AMD, Ni stayed soluble until ~pH 5.5, then began forming precipitates (researchgate.net).
Neutralizing to pH 6.5 in one test cut Ni from 0.75 mg/L to 0.32–0.41 mg/L and reduced Zn from ~4.4 mg/L to 0.3–1.0 mg/L (onlinelibrary.wiley.com). By the end of Stage 2, effluent often carries on the order of <0.5–1 mg/L Ni, subject to influent and dose. A neutralization tank with moderate mixing and a final clarifier typically captures the bulk of mixed‑metal hydroxides (often >2% solids). Where footprint is tight, plants boost settling capacity with tube settlers.
Stage 3: high‑pH nickel precipitation
To target remaining nickel (and potentially manganese), push pH to ≈8–9+. Nickel hydroxide (Ni(OH)₂) solubility drops sharply at higher pH: Ni remains mostly ionic until ~pH 6–7, then precipitates rapidly above ≈8–9 as Ni(OH)₂ (or NiCO₃ with carbonate) (researchgate.net; onlinelibrary.wiley.com). A geochemical simulation shows Ni precipitation onset above pH 8.2 (researchgate.net). Manganese also drops out at pH ≈9–10, though it is often minor in nickel AMD.
This high‑pH step uses rapid mixing and then intermediate settling or filtration; crystalline Ni(OH)₂ sludge (often co‑precipitated with CaCO₃) is dewatered. Because discharge pH must be 6–9, the stream is re‑acidified—commonly via CO₂ injection or mild acid addition—before release (nikel.co.id; link text shows “Cr total: 0.5 mg/L”). Plants often polish the filtered stream with a sand filter to capture residual fines.
Polishing options to guarantee low nickel
Stage 2 alone can land near the standard—one test left ~0.3–0.4 mg/L Ni at pH 6.5 (onlinelibrary.wiley.com)—already within Indonesia’s ≤0.5 mg/L cap (nikel.co.id). But variable influent or tighter limits often justify a polish.
Extended settling or filtration can capture fine Ni hydroxide colloids. Many plants add membrane filters with pore <1 μm; ultrafiltration units serve this role effectively, especially as a compact pretreatment/polish. High‑rate media filtration also fits.
Ion exchange or adsorption can scavenge residual Ni²⁺ to very low levels. A small polishing column with a chelating resin (e.g., iminodiacetate type) is proven in metal finishing effluent control to reach sub‑ppb nickel (mdpi.com). System integrators deploy compact ion exchange skids, then specify the appropriate chelating resin. Alternative adsorbents (activated carbon, manganese oxide, engineered nanoparticles) can target Ni, though industrial experience in mine waters is limited.
A secondary precipitation push (e.g., an additional caustic or soda ash dose to pH ∼11) can drive near‑complete Ni(OH)₂ formation; the stream is then re‑corrected for pH and re‑checked against 6–9 before discharge (nikel.co.id).
Example sizing and doses
Consider 1000 m³/d of AMD at pH 3, acidity ~1 g/L as CaCO₃, Fe 50 mg/L, Al 80 mg/L, Ni 1 mg/L (worst‑case). Stage 1 lime addition (~1000 kg/d) lifts pH to ~4; clarified effluent shows pH ~4.5–5 with Fe/Al ~0.5 mg/L. Stage 2 (~500 kg/d lime) brings pH to ~6.5; Fe/Al fall to <0.1 mg/L, Ni drops ~80% to ~0.2 mg/L. Stage 3 (~200 kg/d) raises pH to 9; Ni precipitates to <0.05 mg/L. After CO₂ re‑acidification, effluent pH ≈7 and Ni <0.1 mg/L. These rough calculations align with Park et al. (2013), who saw ~99% Fe/Al removal and Ni ≈0.32 mg/L at pH 6.5 with Ca(OH)₂ (onlinelibrary.wiley.com; onlinelibrary.wiley.com). Pushing to pH 9 yields even lower residual Ni.
Control logic, residence time, and sludge
Each reactor is sized for 10–30 minutes of reaction/settling; dosing is controlled by pH and mass balances (or by jar‑testing surrogates). Neutralization tanks and settling units are standard, with clarifiers pulling down solids between stages. Sludges are typically returned to a thickener and dewatered; lime sludge is then disposed appropriately.
In short, a staged design—first to <4 for Fe/Al, then to neutral for most metals, and finally to ~9 for Ni—meets Indonesia’s discharge criteria. Hydrated lime is cost‑effective for bulk neutralization (and readily precipitates metals), with caustic soda used for rapid fine control when needed (onlinelibrary.wiley.com; onemine.org). A filtration and/or ion‑exchange polish adds margin for consistently sub‑0.5 mg/L Ni (nikel.co.id).
Sources: U.S. EPA design guidelines and AMD case studies provide pH‑precipitation behavior of Fe, Al, Ni, etc. (onlinelibrary.wiley.com; researchgate.net). Park et al. (2013) quantified Ni removal versus pH with NaOH/Ca(OH)₂ (onlinelibrary.wiley.com; onlinelibrary.wiley.com); Indonesian standards (Permen LH 09/2006) specify Ni ≤0.5 mg/L (nikel.co.id), guiding treatment targets.
