Nickel refiners are winning with precision: stepwise pH control drops iron to ~2 mg/L and strips copper early, then multi‑stage solvent extraction (SX) splits nickel and cobalt with >98% recoveries and >99% purities. Bench and plant data put hard numbers behind the flowsheets.
Industry: Nickel_Mining | Process: Refining
Refining nickel from acidic leach liquors is less alchemy, more exact science. Start at pH ≈1–2, oxidize ferrous iron (Fe²⁺) to ferric (Fe³⁺), and raise the pH in tight steps; impurities fall out while nickel and cobalt stay put. In practice, that looks like nearly 100% iron removal at pH 2.5–3.3 with only ~1–1.8% nickel/cobalt loss, residual Fe pared to ~2 mg/L after a second neutralization, and temperatures held ~90–95 °C to keep solids filterable (researchgate.net).
With copper, the window shifts: hydroxide precipitation clears Cu at around pH 7 while Ni barely budges until ≈10, or copper can be sulfided as CuS at pH ~2 thanks to its vanishing solubility product (Ksp ∼10^–45 vs. NiS at ∼10^–20) (researchgate.net). The payoff is a clean Ni/Co sulfate liquor ready for solvent extraction.
Iron removal by controlled pH
Process engineers begin with oxidants such as H₂O₂ or air to convert Fe²⁺ to Fe³⁺, then add base. One study (Silva et al.) raised an iron‑rich laterite pregnant leach solution (PLS, leach liquor) from pH ~1.6 to 2.5–3.3 using NH₄OH (ammonium hydroxide). At pH 2.5–3.3, nearly 100% of dissolved iron precipitated—mainly as jarosite/goethite (iron sulfate and iron oxyhydroxide solids)—while Ni²⁺ and Co²⁺ losses were only ~1–1.8% (researchgate.net).
The precipitate (mixed K/NH₄ jarosite and goethite) settled well, and a second neutralization with lime (CaO) to pH ~3.7–3.8 pushed residual Fe down to ~2 mg/L, primarily as bassanite/gypsum, with Ni/Co losses still <1.6% (researchgate.net). The two‑stage scheme (ammonia then lime) cleared >99% of iron while preserving >98% of Ni/Co; precipitation temperature was kept high (~90–95 °C) to improve filterability (same source). Where base addition needs tight control at these narrow pH setpoints, plants apply accurate chemical metering equipment such as a dosing pump to track the pH rise closely without overshoot.
When solids settle well, facilities typically move to solid–liquid separation; a clarifier provides a defined detention time to remove suspended precipitates before downstream circuits.
Copper out at pH 7, nickel stays soluble
Copper behaves differently from nickel: its hydroxide solubility is much lower. One study neutralizing a Cu–Ni wastewater found Cu²⁺ precipitating almost completely by pH ≈7, whereas Ni²⁺ only began to precipitate at ≈10 (researchgate.net). In practice, Ca(OH)₂ addition to ∼pH 7 can remove ~100% of copper as Cu(OH)₂ with negligible nickel loss because Ni ≫ Cu solubility (same source). Conversely, Ni²⁺ requires pH ~9–10 to co‑precipitate, so it stays in solution at pH 7 (same source).
Hydrometallurgical nickel refining also uses selective sulfide precipitation: adding a sulfide (e.g., Na₂S/H₂S) at pH ~2 removes copper as CuS (Ksp ∼10^–45) while nickel remains soluble (NiS Ksp ∼10^–20) (researchgate.net). Where settling rates need a boost in these steps, operators often dose polymers; a targeted flocculant increases particle agglomeration and improves separator efficiency without changing the underlying pH chemistry described in the studies.
Stepwise neutralization results
Key outcome across the iron and copper stages: by stepwise neutralization, targeted impurities drop out. Typical results are >99% Fe removal (e.g., to <2 mg/L) with <2% Ni/Co carry‑loss (researchgate.net). Copper, if present, is removed at a higher pH (hydroxide or sulfide precipitation) long before appreciable nickel hydroxide forms (researchgate.net). This leaves a purified Ni/Co sulfate liquor to feed the SX circuit.
Multi‑stage solvent extraction (SX) circuits
After impurity precipitation, Ni²⁺ and Co²⁺ remain together in sulfate solution. Commercial refiners use multi‑stage counter‑current SX (solvent extraction, a liquid–liquid separation) to split them. Two general schemes are standard: (a) Ni‑first circuits that extract nickel into the organic phase then strip it off (producing Ni electrolytes for electrowinning, EW), and (b) Co‑first circuits that selectively extract cobalt, leaving nickel in the raffinate. Both routes achieve very high purities and recoveries when staged in counter‑current.
One charts‑based nickel‑extraction flow (Bulong, Western Australia, 1999–2003) used direct SX on dilute leach liquor (no intermediate cake). The circuit produced a concentrated NiSO₄ strip liquor yielding >99.8%‑pure Ni cathode on EW; overall nickel recovery from PLS to Ni product was ≈98.2%, and cobalt recovery to a separate Co‑rich strip liquor was ≈98.5% (researchgate.net). In operational terms, typical aqueous Ni breakthrough was ~1–2%, and Co breakthrough ~1–2%, demonstrating tight separation (same source).
Co‑selective extraction using Cyanex 272
An alternative starts with a Co‑selective extractant. Cyanex 272 (a phosphinic acid) is noted for its Co/Ni selectivity: at one pH charge (∼7.2), a Nordhoff separation factor of ~8000 for cobalt over nickel was reported (researchgate.net). Kasese Cobalt Ltd. (Uganda) and others built on this: an SX train with Cyanex 272 extracted Co²⁺ (and Mn²⁺), leaving Ni²⁺ in the raffinate; any trace nickel co‑extracted was scrubbed out via a CoSO₄ wash (researchgate.net). Subsequent stripping gave a pure cobalt liquor and a nickel‑rich raffinate. A follow‑up 3‑stage D2EHPA extraction then separated Mn²⁺ from Co²⁺, yielding final raffinate streams of ~99.9%‑pure Co, Mn, and Ni (same source). In short, consecutive SX (Cyanex 272 step + D2EHPA step) delivered essentially 100% pure Ni²⁺ and Co²⁺ products (same source).
D2EHPA, Versatic/LIX, and selective stripping
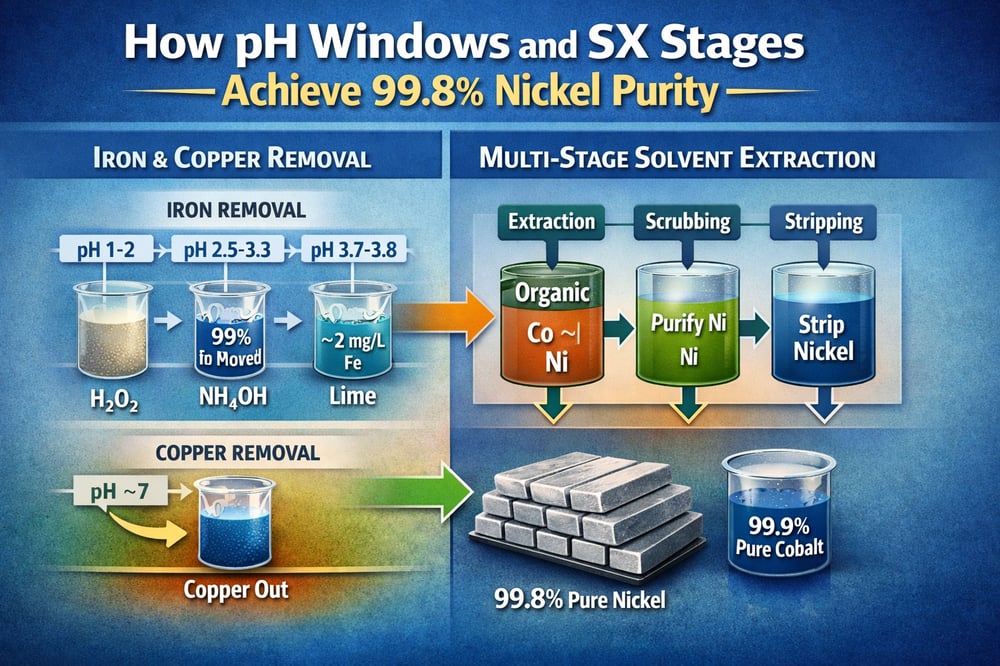
Other circuits rely on D2EHPA (di‑(2‑ethylhexyl) phosphoric acid) or Versatic 10/LIX systems where Ni and Co co‑extract initially (often at pH ~3–5), then split by staged stripping. In an ammoniacal LIX example for Ni & Cu SX, >99% nickel extraction was achieved in 3 stages, then >99.9% nickel strip in five stages (redesign.911metallurgist.com). In a strong acid/sulfate system, a multi‑stage counter‑current SX with 1 M D2EHPA can extract essentially all Ni and Co; conventional strip stages (first Ni, then Co) yield separate high‑purity products. In practice, design data confirm the approach: Ni recoveries to final Zn‑free Ni electrolyte routinely exceed 98%, and Co recoveries exceed 98%; product purities are ≥99%—for example, Ni cathode >99.8% Ni and theoretical Ni solutions >99.9% pure (researchgate.net; researchgate.net).
Across these SX schemes, pH control is pivotal: too high and co‑precipitation or co‑extraction of impurities occurs; too low and target metals are left behind. Extractant dosing in cascaded stages typically runs 1–20 vol% organics, adjusted to meet breakthrough and strip targets described in the plant and bench data. Reagents mentioned across the flows—ammonia (NH₄OH) for jarosite‑mode iron removal, lime (CaO) for final clean‑up, and sodium sulfide for CuS precipitation—are part of standard hydromet circuits; a supplier with a complete range of water and wastewater chemicals helps align availability to the pH windows cited. The process targets clean‑metals performance compatible with downstream electrowinning or saleable sulfate products and operates under regulatory constraints (e.g., Indonesian discharge pH 6–9).
Figures and metrics recap
- >99% Fe removal (e.g., to <2 mg/L) at pH 2.5–3.3 (researchgate.net)
- <2% Ni/Co loss during Fe precipitation (researchgate.net)
- >98% Ni recovery (to Ni product) and >98% Co recovery in SX (researchgate.net)
- Ni strip yields at 99.9% of loaded Ni in multi‑stage SX (redesign.911metallurgist.com)
- Ni cathode purity >99.8% and Co/Mn stream purity >99.9% (researchgate.net; researchgate.net)
All of this hinges on nailing the chemistry as documented: oxidize Fe²⁺, track pH precisely through 2.5–3.3 and ~3.7–3.8, keep precipitation hot (~90–95 °C), clear copper around pH 7 or via sulfide at pH ~2, and run multi‑stage SX with disciplined pH and organic loading. Where operations need supporting equipment around these steps—from accurate metering to solid–liquid separation—the choices slot in behind the chemistry rather than define it.
Sources for the figures and flows discussed include peer‑reviewed studies and industry reports: researchgate.net, researchgate.net, researchgate.net, researchgate.net, researchgate.net, and redesign.911metallurgist.com.
