In the ever-growing industrialized world, water and waste treatment technologies have become very important. Amidst environmental challenges and the need for higher efficiency, various technological innovations have emerged. One technology that plays an important role is electroplating, a process that not only improves product quality, but also helps in water and waste treatment. Electroplating, historically developed for industrial purposes such as manufacturing and jewelry, has now found vital applications in ensuring sustainability and efficiency in water resource management.
The importance of electroplating in modern industry cannot be underestimated. According to research by agencies such as the Environmental Protection Agency (EPA) and other international organizations, the electroplating process has proven effective in reducing toxic waste, reducing the use of hazardous chemicals, and increasing water and waste recycling. With its ability to treat and recycle materials more efficiently, electroplating plays a key role in the industry's sustainability efforts.
This article will explain how electroplating is used to reduce toxic waste, reduce the use of hazardous chemicals, and increase water and waste recycling.
This article will explain how electroplating is becoming an essential part of modern treatment systems. We will dive into how electroplating works, its applications in water treatment, its contribution to effective waste management, as well as recent innovations that bring this technology to the forefront of environmental solutions. Through in-depth explanations, readers will gain a better understanding of electroplating's crucial role in maintaining the balance between industrial needs and environmental responsibility.
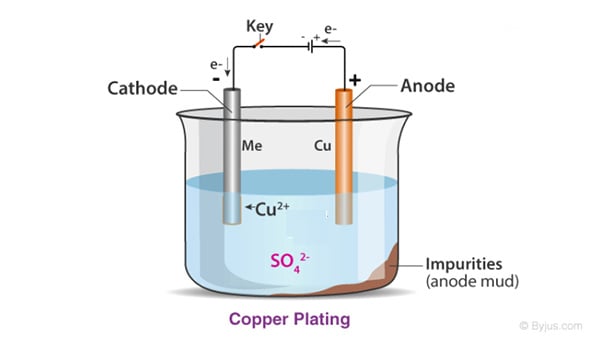
Definition of Electroplating
Electroplating is the process of coating metal on an object using an electric current. This process, also known as electrodeposition, works on the principle of an electrolytic cell. In this process, positive metal ions in an electrolyte solution, such as copper sulfate for copper plating, are reduced to metal at the cathode. For example, Cu^2+ ions are reduced to metallic copper at the cathode. Conversely, at the anode, the opposite reaction can occur, converting the metal into dissolved ions. This enables the effective transfer of metal from the anode to the cathode.
.
The electroplating process has evolved over time and can currently be categorized into several main methods, such as bulk plating, rack plating, continuous plating, and in-line plating. Each of these methods is customized for different applications. For example, bulk plating, such as barrel plating, is efficient for mass production of small parts such as nuts and bolts, while rack plating is suitable for larger and complex parts that require higher coating quality
.Electroplating not only improves the appearance of objects, but also improves their physical and mechanical properties. This process can increase corrosion resistance, tensile strength, and wear resistance, as well as improve conductivity and surface finish. In addition, electroplating also allows the coating of non-conductive materials, such as plastics and wood, after they have been treated to become conductive.
However, the electroplating process is not as efficient as it could be.
However, the electroplating process also has its limitations. For example, the process can be complex and difficult to perform consistently, and its benefits are limited to surface coatings. In addition, the process can also produce harmful gases due to reduction at the cathode
.
Electroplating in Water Treatment
Electroplating in water treatment focuses on using electroplating technology to improve water quality, which can include removing harmful contaminants and improving certain aspects of the treated water. One of the main applications of this technology in water treatment is for the removal of heavy metal contaminants, which can include chromium, lead, arsenic, and other heavy metals.
The methods used in electroplating in water treatment focus on using electroplating technology to improve water quality, which can include removing harmful contaminants and improving certain aspects of treated water.
The methods used in electroplating for water treatment include:
- Electrocoagulation: This technique involves the use of electric current to convert metal contaminants into a form that is easier to separate from water. It is effective for removing suspended particles, heavy metals, and other contaminants from water.
- Electrocoagulation.
- Electrofloccuation: Similar to electrocoagulation, electrofloccuation uses an electric current to produce a flocculant that can attract and bind to contaminants, thus easing the process of separating contaminants from water.
- Electrofloccuation.
- Electrochemical Oxidation: This method uses electrodes to induce oxidation reactions that can decompose organic and inorganic contaminants in water.
- Electrochemical Oxidation.
- Ion Exchange and Electrodeionization: These techniques involve the exchange of contaminant ions with other, less harmful ions, or the use of electricity to separate ions from water.
- Electrodeionization .
The use of electroplating technology in water treatment offers several advantages, including the ability to treat water with high efficiency, reduce the use of harmful chemicals, and provide a more environmentally friendly solution compared to traditional water treatment methods.
However, it is important to note that electroplating technology can be used to treat water with high efficiency. <however, it="" should="" be="" noted="" that="" this="" technology="" also="" has="" limitations="" and="" challenges,="" including="" operational="" maintenance="" costs,="" as="" well="" the="" need="" for="" strict="" control="" over="" conditions="" to="" ensure="" treatment="" effectiveness="" efficiency.<="" p=""> </however,>

Electroplating in Wastewater Treatment
In electroplating wastewater treatment, there are various methods used, such as chemical reagent method, electrochemical method, and ion exchange method. The chemical reagent method involves converting heavy metal ions into insoluble hydroxides through chemical reactions, followed by precipitation and separation. Although this method is simple and common, there are some drawbacks, including high reagent consumption and a slow process.
Electrochemical methods, electrochemical methods, and ion exchange methods.
Electrochemical methods, including electrocoagulation and galvanic coagulation, are commonly used to remove hexavalent chromium ions from wastewater. The process involves dissolving iron which then reduces hexavalent chromium to trivalent chromium forming hydroxides. This method is quite complex and its duration depends on the pH of the media, the intensity of the current, and the duration of the wastewater treatment.
Ion exchange methods are based on the ability of cation and anion exchangers to absorb ions from aqueous solutions. Once saturated, these exchangers are regenerated to restore their ion exchange capability. However, this method has limitations such as excessive use of reagents and high water consumption for leaching of ion materials.
Ion exchange methods are based on the ability of the exchanger to absorb ions from the water solution.
In addition to these methods, there is also a new approach that uses rotating electromagnetic fields with ferromagnetic particles. This approach can improve the efficiency of the reagent method by reducing reagent consumption and reaction time, while also saving energy.
Thus, wastewater treatment is an important part of the treatment process.
Thus, electroplating wastewater treatment is a vital and complex process, involving various methods to remove various harmful contaminants, in order to protect ecosystems and human health.
Current Innovations and Trends
Current innovations and trends in electroplating technology focus on developing more effective, cost-effective, and sustainable methods. Some notable innovations include:
- The use of Hydrophobic and Hydrophilic Paints on Pre-painted Metal: This technology allows the creation of paint coatings that repel or attract water, improving the functionality and durability of the metal.
- Nanotechnology Coating: This method uses extremely thin coatings at the nanoscale to improve durability without affecting the aesthetics or thickness of the material.
- Nanotechnology Coating.
- Environmentally Friendly Electroplating: Development of electroplating methods that use materials that are less harmful to the environment, such as the use of water as a solvent and avoiding wet processes.
- Graphene Coating: Using graphene for metal coatings increases strength, electrical and thermal conductivity, and corrosion resistance.
- Graphene Coatings
- Data Analytics: The use of data analytics to improve the efficiency of electroplating processes, enabling the discovery of new solutions to existing problems.
- Data Analytics.
Sustainability and efficiency improvements are a key focus in electroplating innovation, creating new opportunities for applications in a wide range of industries.

Environmental Impact and Sustainability
Electroplating, while it has many benefits, also carries significant environmental impacts. The process often uses hazardous chemicals and can generate waste that can potentially damage ecosystems if not handled properly. Therefore, it is imperative to implement sustainability regulations and practices in the electroplating process. This includes the use of cleaner technologies, reduced use of hazardous chemicals, and effective waste treatment and recycling. Through this approach, electroplating's negative impact on the environment can be minimized, allowing the industry to operate in a more sustainable and environmentally-friendly manner.
Closing
Electroplating, as an important part of water and sewage treatment, has a key role in maintaining the balance between industrial needs and environmental protection. Through continuous innovation and development, electroplating technology enables increased efficiency and reduced environmental impact. By focusing on sustainability practices and complying with environmental regulations, electroplating not only supports industry, but also contributes to efforts to maintain the health of our planet. This realization is pushing towards a future where technology and sustainability can go hand in hand, resulting in more environmentally friendly and sustainable solutions for future generations.
To explore more about innovative and eco-friendly water and sewage treatment solutions, visit Beta Pramesti Asia's website. We offer a diverse selection of advanced solutions that are customized to meet your specific needs. Our team is ready to help you find the most effective and sustainable solution, which not only meets the needs of the industry but also contributes to the protection of the environment.
