In the water and wastewater treatment process, the use of neutralizing cleaning chemicals plays a role that cannot be ignored. This neutralization process is important to ensure the quality of water produced is in accordance with applicable safety standards and regulations. This article will provide an overview of 'Neutralizing cleaning chemicals for water and wastewater treatment'. We will discuss what neutralizing cleaning chemicals are, their function in water treatment, and how important these processes are in supporting efficient and sustainable water treatment. By understanding their vital role, we can better appreciate their contribution in maintaining public health. In the water and wastewater treatment process, the use of neutralized cleaning chemicals plays a role that cannot be ignored.
This neutralization process is important to ensure the quality of the water produced complies with safety standards and applicable regulations. This article will provide an overview of 'Neutralizing cleaning chemicals for water and wastewater treatment'. We will discuss what neutralizing cleaning chemicals are, their function in water treatment, and how important these processes are in supporting efficient and sustainable water treatment. By understanding their vital role, we can better appreciate their contribution in maintaining public health and the environment. Furthermore, we will also explore how the latest innovations and technologies in this field contribute towards increasing efficiency and reducing negative impacts on the environment, highlighting the importance of sustainable development in the water treatment industry.
 Figure>.
Figure>.What are Neutralizing Cleaning Chemicals?
The use of these chemicals not only optimizes the treatment process but also ensures that the water released into the environment is safe and does not harm the ecosystem. In addition, neutralization also contributes to reducing corrosion of treatment equipment, extending equipment life, and reducing maintenance costs.
In industrial wastewater treatment, for example, neutralization is often required to treat effluents containing strong acids or bases from production processes. This neutralization process helps stabilize the effluent so that it can be further processed or safely disposed of. In addition, neutralization is also important in drinking water treatment, where the pH of the water must be kept within a certain range to prevent pipe corrosion and maintain water quality for consumption. With the proper selection and use of cleaning chemicals, the neutralization process becomes a key step in ensuring the water we use and dispose of is not only safe for humans, but also for the environment.
Why is Neutralization Important in Water Treatment?
Neutralization in water treatment is a critical step that aims to protect the environment and meet safety standards. This process is important because:
 Figure>.
Figure>.- Environmental Impact
Unneutralized water can damage local ecosystems, harming flora and fauna. It can cause major changes in biological balance, threatening vulnerable species and disrupting food chains. For example, water pH that is too acidic or alkaline can cause mass die-offs of aquatic organisms such as fish, shrimps and other riverine life. This not only affects those species directly, but also predators and other species that depend on them for food.
In addition, changes in pH can affect the availability of nutrients and heavy metals in water. Nutrients such as phosphorus and nitrogen can become more soluble under certain pH conditions, causing excessive algae growth resulting in eutrophication. This eutrophication reduces oxygen levels in the water, threatening aquatic life and compromising water quality. On the other hand, pH extremes can also cause heavy metals such as lead and mercury to become more soluble, increasing the risk of pollution and accumulation in the food chain. The accumulation of these heavy metals is not only harmful to aquatic life but can also enter the human food chain, causing long-term health problems.
These impacts show how important neutralization in water and wastewater treatment is, not only to protect aquatic life but also to maintain overall ecosystem health.

- Regulatory Compliance
Government regulations require neutralization in wastewater treatment to prevent pollution. Conformance to these standards is not only important for legal compliance, but also for maintaining a company's reputation and operations. These regulations often include specific limits for the pH of wastewater that can be discharged into the environment, ensuring that companies make sufficient efforts to reduce negative impacts on the environment.
Compliance with these regulations also reflects a company's social responsibility and their commitment to sustainable environmental practices. Violations of these standards can result in legal sanctions, fines, and significant reputational damage, all of which can negatively impact consumer confidence and company credibility with the public and stakeholders.
In addition, these regulations are frequently updated and become more stringent as environmental awareness and water treatment technologies evolve. Therefore, companies need to keep abreast of the latest developments in regulatory standards and water treatment technologies to ensure that they not only meet current standards but are also prepared to adapt their operations to future regulatory changes.
Understanding and proper application of neutralization in wastewater treatment is becoming key to ensuring companies not only comply with the law but also demonstrate leadership and innovation in responsible environmental practices.
 Figure>.
Figure>.- Public Health
Water with an unbalanced pH can contain heavy metals and other harmful substances that dissolve in water. Neutralization helps reduce these health risks, keeping water safe for household and industrial use. Extreme pH conditions, either too acidic or too alkaline, can facilitate the dissolution of harmful substances such as arsenic and mercury, which are highly toxic even in small amounts. This can lead to a variety of long-term health problems, including organ damage, neurological disorders, and an increased risk of cancer.
Neutralization also reduces the risk of microbiological contamination. Water with an unbalanced pH can disrupt biological treatment processes that normally eliminate pathogens. This increases the risk of waterborne diseases such as hepatitis, gastroenteritis and parasitic infections. Maintaining pH balance is key to optimizing these treatment processes and ensuring safe water from microbiological contaminants.
Furthermore, a balanced water pH is important to avoid unwanted taste and odor changes. Water with an inappropriate pH can have a metallic or very bitter taste, which is not only unpleasant but can also indicate the presence of contamination. Neutral drinking water provides safety and comfort to consumers.
Overall, neutralization in water treatment is not only important to remove chemical and biological contaminants, but also to provide water that is safe and convenient for daily use. This step plays a vital role in ensuring that the water we consume and use in our daily lives meets strict health standards and is free from unnecessary health risks.
 Figure>.
Figure>.- Process Efficiency
Netralization supports other water treatment processes such as coagulation, flocculation, and sedimentation, increasing the overall effectiveness of water purification. The neutralization process helps adjust the pH of the water so that it is conducive to these processes. For example, in the coagulation process, an optimal pH is required for the coagulant to work effectively in agglomerating small particles that cannot be filtered or settle on their own. If the pH is too acidic or alkaline, the effectiveness of the coagulant will be reduced, thereby reducing the efficiency of water treatment.
Furthermore, in the flocculation process, the right pH allows the particles that have been coagulated by the coagulant to form larger flocs and are easier to settle or filter. An unbalanced pH can inhibit the flocculation process, causing particles to remain dispersed in the water and reducing the quality of purification.
Sedimentation, the process by which particles settle to the bottom of a tank, also relies heavily on balanced pH conditions. If the pH of the water is too extreme, particles may remain floating or not settle efficiently, reducing the effectiveness of this process in removing contaminants.
In addition, neutralization is also essential to the disinfection process. A balanced pH ensures that disinfectants such as chlorine work to their full potential. An unbalanced water pH can reduce the effectiveness of chlorine in killing harmful microorganisms, leaving behind unwanted health risks.
Thus, neutralization is not only essential for producing chemically and microbiologically safe water, but it also plays a vital role in ensuring that other water treatment processes work with maximum effectiveness. This step contributes significantly to achieving efficiency and effectiveness in water treatment systems.
 Figure>.
Figure>.- Corrosion Prevention
Keeping the pH of the water at a stable level is also important to prevent damage to infrastructure and equipment. This significantly reduces operational costs and long-term maintenance investments. Water with extreme pH, either too acidic or too alkaline, can cause rapid corrosion of pipes, tanks and other metal components used in water treatment systems. This corrosion not only reduces equipment lifespan but can also lead to leaks or unexpected system failures, which can result in costly operational disruptions and potential safety risks.
In addition, corrosion can lead to contamination of the treated water. For example, corrosion of copper pipes can release copper particles into the water, which not only affects water quality but is also harmful to human health if consumed. In the case of iron or steel pipes, corrosion can lead to the release of iron particles that cause discoloration and taste of the water, as well as disrupt further treatment processes.
Neutralization helps optimize the pH of water, thereby reducing the potential for corrosive reactions. This is important not only in wastewater treatment processes but also in drinking water distribution systems. By keeping the pH of the water within a safe range, water treatment companies can avoid the high repair and replacement costs associated with corrosion damage.
In addition, corrosion control through neutralization is part of preventive maintenance practices. By taking proactive steps to manage water pH, companies can prevent damage rather than deal with the costly consequences of corrosion that has already occurred. This not only saves costs but also ensures the reliability and operational sustainability of the water treatment system.
Therefore, neutralization is not only an important step in ensuring water quality but also an important strategy in asset management and infrastructure maintenance in the water treatment industry.
 Figure>.
Figure>.- Hazardous Waste Reduction
By neutralizing hazardous chemicals, this process reduces the amount of hazardous waste that must be treated and disposed of, reducing the burden on sewage treatment facilities and waste disposal systems. Neutralization processes help convert hazardous and reactive chemicals into more stable and less toxic forms. For example, neutralization of hazardous acids and bases produces salt and water, which are much safer to treat and dispose of.
In addition to reducing health and environmental risks, this hazardous waste reduction also reduces operational costs. Hazardous waste treatment and disposal often require specialized and expensive technology. By reducing the amount of hazardous waste generated, companies can save costs on this process.
The neutralization process also helps companies comply with strict environmental regulations regarding hazardous waste disposal. By reducing the toxicity of waste, companies can more easily meet regulatory standards, avoid fines and penalties, and maintain a good environmental reputation.
In addition, hazardous waste neutralization also contributes to sustainability initiatives and environmental footprint reduction. By reducing the amount of hazardous waste generated, companies demonstrate their commitment to responsible and sustainable business practices.
Therefore, neutralization is not only an important step in water and sewage treatment, but also a key strategy in hazardous waste management, supporting environmental protection and sustainability efforts.
 Figure>.
Figure>.- Adaptation to Water Source Variability
Source water can be variable in chemical composition. Neutralization helps water treatment to adjust to these variations, ensuring consistency in the quality of water produced. This variability can come from a variety of factors, such as seasonal changes, industrial activity in upstream areas, or agricultural run-off that carries fertilizers and pesticides into the water source. These changes can affect the water's pH, mineral content, and the presence of chemical contaminants.
Using the neutralization process, water treatment facilities can effectively adjust the pH condition of the water according to the needs of the subsequent treatment process. For example, water coming from industrialized areas may have a more acidic pH due to industrial pollution, requiring pH adjustment before further treatment processes can be carried out.
In addition, neutralization is also important in anticipating seasonal changes. During the rainy season, for example, the water entering the treatment facility may be more acidic due to dissolved acids from the rain. On the other hand, during the dry season, the concentration of minerals and other contaminants in the source water may increase, requiring different adjustments.
Neutralization also helps in reducing the effects of contaminants that can change according to pH, such as heavy metals whose solubility may increase at certain pH. By adjusting the pH, this process helps reduce the risk of these contaminants becoming a problem in further water treatment.
Therefore, neutralization is an important step in responding to source water variability, ensuring that the quality of treated water is consistent and safe for subsequent use, whether for human consumption, industrial use, or release back into the environment.
Common Neutralization Agents Used
Some commonly used neutralization agents include:
- Hydroxide of Sodium (NaOH): Also known as caustic soda, it is very effective at raising the pH of acidic water. It reacts with acids in water to form salts and water, which helps neutralize the pH. Hydroxide of Sodium is often used in industry and water treatment facilities because it dissolves easily and provides fast results.
- Sulfuric Acid (H2SO4): Used to lower the pH of water that is alkaline. It is a strong acid that reacts with bases in water to form a more neutral salt. Sulfuric Acid is highly effective and economical, making it a popular choice in many water treatment applications.
In addition to these two agents, there are also other ingredients that are often used in neutralization, such as:
- Lime (Ca(OH)2): Known as calcium hydroxide, lime is used to neutralize acidic water, especially in wastewater treatment. In addition to raising the pH, lime also helps remove heavy metals and phosphates from water.
- Carbonic Acid (H2CO3): Used in rarer scenarios, carbonic acid can lower the pH of highly alkaline water. It is often formed naturally in water when dissolved carbon dioxide reacts with water.
- Citric Acid: This weak organic acid is used for finer pH adjustments, especially in applications where very specific pH control is required.
Each of these agents is unique in how they work, and the selection of the right agent depends on the characteristics of the water being treated. Variables such as the strength of the acid or base that needs to be neutralized, the presence of certain contaminants, as well as cost and material availability considerations, all influence the choice of neutralization agent. It is also important to consider the safety and handling aspects of these chemicals, as some of them can be highly corrosive or dangerous if not handled properly.
 Figure>.
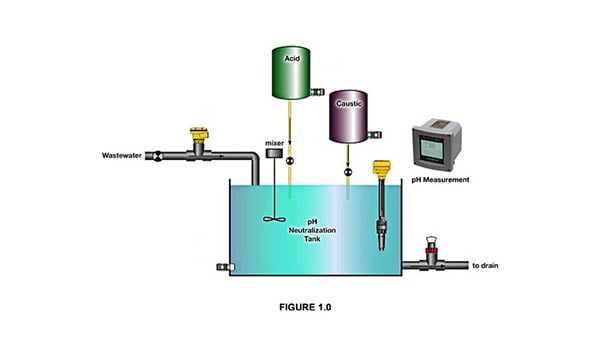
Figure>.The Neutralization Process in Water Treatment
The neutralization process involves several key steps, including the initial assessment of the water's pH, the addition of an appropriate neutralization agent, and the re-measurement of the pH to ensure that the water has reached the desired balance. This process is not only important for water quality but also for the overall efficiency of the water treatment system.
- Initial pH Evaluation: Before adding neutralization agents, it is necessary to know the pH level of the water. This is done using a pH measuring device such as a pH meter or litmus paper. This measurement provides initial information on how acidic or alkaline the water needs to be treated.
- Agent Addition: Depending on the initial pH, a base or acid will be added. The amount and concentration of the added agent should be carefully calculated to avoid over-correction, which can lead to a pH change that is too far in the other direction.
- Stirring and Contact Time: After addition, the water is usually stirred or circulated to ensure good mixing. Sufficient contact time allows the chemical reaction to take place effectively and evenly throughout the volume of water.
- Remeasurement: After addition and sufficient contact time, the pH of the water is remeasured to ensure the effectiveness of the process. If necessary, additional adjustments can be made to achieve the desired pH balance.
Case example: A water treatment facility uses Sodium Hydroxide to neutralize acidic wastewater from industrial processes. After addition, they stirred the wastewater to ensure even mixing, then measured the pH again. If the pH still did not reach the desired level, they would add more Sodium Hydroxide and repeat the process until it reached the right pH.
This process ensures that the treated water is not only safe for the environment and human health but also optimal for advanced treatment processes. Proper neutralization increases the effectiveness of water treatment and reduces the risk of problems caused by unbalanced pH.
Conclusion
Chemical neutralization in water and wastewater treatment is a very important process. Through understanding and implementing effective neutralization methods, we can ensure that treated water not only meets safety and environmental compliance standards but also contributes to ecosystem sustainability. This process, integrated in 'Neutralizing cleaning chemicals for water and wastewater treatment', demonstrates our commitment to environmental protection and public health.
In addition, neutralization plays an important role in maintaining ecological balance and preventing ecosystem damage due to pH imbalance. It also supports public health by reducing risks related to harmful contaminants that can be dissolved in water with extreme pH. With this risk reduction, neutralization helps in providing safe water for consumption and industrial activities, ensuring that basic human needs and economic activities can continue without harming the environment.
In the context of sustainable water resources management, neutralization is not only a technical step in water treatment, but also part of a broader solution to address environmental challenges. Through the use of this technique, we affirm our commitment to environmental sustainability and sustainable development, ensuring that current and future generations have access to clean and safe water.
The neutralization process, therefore, becomes key in our efforts to create a healthier and more sustainable world, where water resources are managed in a responsible and effective manner for the good of all.
For more information about water treatment and the use of neutralized cleaning chemicals, contact us via Whatsapp or e-mail. We are committed to providing sustainable and effective water treatment solutions.
